Episode 111: Akathisia
By listening to this episode, you can earn 1.25 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Annabel Kuhn, BA, Michael A. Cummings, MD, David Puder, MD
Corresponding author: David Puder, M.D.
No author or presenter has any conflicts of interest.
In this episode of the podcast we discuss akathisia, the horrible and all too common side effect of psychiatric medications. Subsequently, we go through definitions, history, mechanism, how to rate it, and treatment.
The Definition of Akathisia:
Akathisia is defined as a feeling of restlessness and an urgent need to move. Discomfort in the muscles, often described as jitteriness or tension. The lower extremities are the most often affected.
Subjective symptoms include inner tension, anxiety, panic, irritability, discomfort, and sleeplessness (Patel & Marwaha, 2020). On physical examination, irresistible leg movements, difficulty sitting and standing, rubbing or rocking while sitting, vocalizations such as grunting or moaning, or repetitive movements may be seen (Patel & Marwaha, 2020).
Along with tension, anxiety, motor restlessness, hyperkinesis, pacing, lower extremity discomfort there is insomnia, mood lability, panic and suicidality (Heally, 2006).
“Although commonly considered a type of movement disorder or EPS, in fact, akathisia should be considered more a sensorimotor disorder because of the powerful sensory component, which is a defining characteristic of the condition. In fact, the sensory component may be the primary problem, with the motor signs being secondary to restlessness and need to move,” (Lohr 2015 pg 5).
“Akathisia is commonly observed after treatment with first-generation antipsychotic medications with reports of prevalence in the range of 8-76% of treated patients, making it arguably the most common side effect of these medications,” (Lohr 2015 pg 6).
What is the History of Akathisia?
Ladislav Haskovec (1866-1944)
Described it in 1901 in two patients with Parkinson’s disease as anxiety and motor restlessness, discomfort in legs and knees and transiently relieved by weight-bearing and walking (Mohr, 2002)
Jack Henry Abbot (1981)
“These medications attack from so deep… the pain grinds into your fiber… you ache with restlessness….” In the Belly of the Beast (ISBN 0-679-73237-3).
Types and Characteristics of Akathisia
Acute and subacute akathisia
“Akathisia usually occurs within a few days to weeks of initiating an antipsychotic medication or increasing the dose. During the initial weeks it is considered acute, and later it is considered subacute,” (Lohr 2015 pg 5).
Acute akathisia responds rapidly to withdrawal of offending agent or condition
Almost all cases of acute akathisia will respond to treatment.
Subacute akathisia has a mixed response to treatment.
Chronic Akathisia
“Chronic akathisia simply refers to akathisia that has been present a long time, usually several months or more, and is a different concept from tardive akathisia,” (Lohr 2015 pg 5).
It is difficult to treat chronic akathisia.
The response rate to treatment (either by discontinuing the offending agent or treatment with medication) is far lower in chronic akathisia than acute akathisia.
Withdrawal akathisia
“This condition, indistinguishable phenomenologically from acute akathisia, occurs upon dosage decrease or withdrawal of antipsychotic or other causative agents. Withdrawal akathisia usually appears within about 2 weeks of discontinuation and disappears within about 6 weeks. If it lasts longer, then it probably represents tardive akathisia,” (Lohr 2015 pg 5).
It has been suggested that akathisia is a direct consequence of dopamine antagonism, however it is likely that there is a more complicated pathophysiological explanation involving derangements and imbalances among GABA, dopamine, serotonin and noradrenergic signaling.
Tardive Akathisia
“Tardive akathisia is indistinguishable in clinical appearance from acute akathisia, but its time of onset and course resembles those of tardive dyskinesia,” (Lohr 2015 pg 5).
Hallmarks of tardive akathisia:
Occurs late in the course of treatment with antipsychotics (usually after 3 months or more)
May emerge initially after antipsychotic discontinuation or dosage reduction
Can often be reduced in severity by INCREASING the antipsychotic dosage (similar to withdrawal akathisia)
For patients with tardive dyskinesia, a short-term increase in dopamine dopamine blockade will decrease symptoms of tardive dyskinesia.
The same is true for tardive akathisia, in which a short-term increase of antipsychotic may decrease restlessness. However, the syndrome tends to reassert itself and is even more persistent and stubborn if the antipsychotic or other causative agent is continued.
“May persist from months to years, even in the absence of medication,” (Lohr 2015 pg 5).
Tardive akathisia is known to be difficult to treat and is minimally responsive to the medications used to treat acute akathisia.
This may represent a loss of neuroplasticity and essentially results in a permanent derangement in the balance among different neural signals.
Pseudoakathisia
“This was a term invented to describe a condition in which there are the objective signs of akathisia without the subjective component. Because akathisia occurs commonly in patients with psychiatric disorders such as schizophrenia, it is not clear if pseudoakathisia should actually be considered a ‘pseudo’ form of the disorder, because many patients experience discomfort that they are unable to understand or express clearly,” (will discuss later in the section “Why is it underdiagnosed?”) (Lohr 2015 pg 5-6).
Most likely still represents true akathisia:
Some individuals living with schizophrenia may have a loss of connection between discomfort and affective response to it. Other individuals may have a deficit in cognitive processing of the discomfort. In each of these cases, it may be difficult for these individuals to express “inner restlessness,” however, phenotypically they demonstrate objective motor symptoms seen in acute and subacute akathisia.
Additionally, it tends to respond to the same treatments and to the same degree as acute and subacute akathisia.
Bing-Sicard akathisia
“This is a term that has been used to describe the occurrence of akathisia in parkinsonian disorders such as Parkinson’s disease and post-encephalitic parkinsonism,” (Lohr 2015 pg 6).
Causes of Akathisia:
Antipsychotic-induced akathisia
First-generation antipsychotics cause akathisia more often than second-generation (Lohr 2015 pg 6).
Highest risk second-generation antipsychotics: aripiprazole and risperidone
Risperidone binds tightly to D2 receptor
Aripiprazole is a partial agonist with a very strong affinity to D2 receptors
Lowest risk second generation antipsychotics: quetiapine, iloperidone, and clozapine
Less robust dopamine antagonists (less tightly bound to dopamine receptors)
According to a 2015 meta-analysis, of the second generation antipsychotics, lurasidone, asenapine, and aripiprazole had significantly higher risk of akathisia compared to placebo and other second generation antipsychotics (Thomas 2015).
RR (95% confidence limits) for lurasidone was 2.7, asenapine was 2.22, and aripiprazole was 1.52.
“Data showed that the relative risk (RR) of akathisia was double that of controls, with lurasidone having the highest individual RR at 2.7 [CI: 2-3.6]. Sensitivity analysis changed the RR of akathisia to less than 10%. The RR of akathisia was still elevated (1.75 [1.4-2.1]) when these drugs were compared only to actives (older SGAs),” (Thomas 2015).
According to a different 2015 study, “clozapine and quetiapine were preferred as the safest drugs in terms of extrapyramidal symptoms or akathisia,” (Oh 2015).
Quetiapine was found to be significantly less likely to induce akathisia compared to aripiprazole (OR=0.46, 95% CI=0.23-0.91) and to risperidone (OR=0.50, 95% CI=0.30-0.78).
Interestingly, the high rate of akathisia in lurasidone virtually vanished when it was recommended to change BID dosing to dosing once in the evening. It is optimally given 30 minutes after a dinner of at least 350 kcal. Lurasidone requires active transport in order to be absorbed. If taken on an empty stomach, it cuts absorption by roughly 50%.
By giving a patient an antipsychotic medication only at night, the patient will experience far fewer EPS/neurologic syndromes because the peak of the drug occurs during sleep. The basal ganglia and motor system are part of the reticular activating system and are basically non-responsive during sleep.
In general, the brain is far more tolerant of gradual change than rapid change. A lower and slower titration will have lower prevalence of adverse effects than a rapid titration of the same medication.
Multiple doses throughout the day also increases adverse effects.
A long-acting injectable form of an antipsychotic is less likely to cause akathisia than its oral version.
Anti-dopaminergic antiemetics
Such as prochlorperazine, and metoclopramide (Lohr 2015 pg 6, and Chauhan, 2012)
Compazine
Reserpine and tetrabenazine
These dopamine-depleting agents (impede reuptake of dopamine into synaptic vesicles) have also been reported to cause akathisia (Lohr 2015 pg 6).
Antidepressants
“SSRIs have received the most reports of an association, with relatively fewer reports of tricyclic antidepressants or MAOIs” (Lohr 2015 pg 6).
One observational study (n=1250) showed that among 58 different antidepressants, duloxetine had an increased relative odds ratio for akathisia (1.15) (Revet et al., 2020).
Serotonin plays a major role in the modulation of the basal ganglia. There are four major dopamine circuits in the brain: mesolimbic, mesocortical, nigrostriatal, and tuberoinfundibular pathways. Of these four, the mesolimbic pathway is the only one which does not have serotonin receptors.
The other three pathways have 5HT2A receptors on cell bodies of the dopamine neurons. If the 5HT2A receptors are occupied by serotonin, it decreases dopamine release by 30-50%. The dopamine signal actually declines rather dramatically.
From the standpoint of the basal ganglia, that is a similar effect to blocking postsynaptic dopamine receptors and decreasing dopamine signal.
Other medications:
Antiepileptics, anticholinergics, sympathomimetics, calcium channel blockers, lithium, anti-parkinson drugs (Duma & Fung, 2019; Sachdev, 1995), and Azithromycin (Riesselman 2015)
Each of these medications affects GABA, norepinephrine, dopamine or serotonin signalling. Anything that disturbs the balance among this interplay of transmitters can cause akathisia.
Recreational drugs:
Gamma-hydroxybutyrate (GHB), methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), and cocaine (Asser 2015)
Acutely when people take these drugs, there is a massive increase in dopamine output, to the point where there is dopamine depletion in the synapse.
Withdrawal states:
Akathisia “can occur in the setting of therapy with other drugs such as SSRIs or in withdrawal states from opiates, benzodiazepines and other drugs,” (Morgan 2019 Therapy of Movement Disorders: A Case-Based Approach, pg 298).
Gabapentin withdrawal (See, 2011)
Benzodiazepines
Benzodiazepines are a treatment for akathisia, but there have been reports of paradoxical akathisia caused by clonazepam, clorazepate, and lorazepam, in patients with traumatic encephalopathy and seizure disorders (Joseph 1993).
Conditions that worsen akathisia:
Renal disease, diabetes, hyperthyroidism, iron deficiency anemia, Parkinson’s disease, and peripheral neuropathy (Salem et al., 2017).
How Quickly Do You See the Symptoms?
Acute akathisia usually presents in days to weeks of starting pharmacotherapy or increase in its doses (Salem et al., 2017). Chronic akathisia and tardive akathisia may present months after initiation or dose increase. Therefore, temporal information is very helpful when working up causes for akathisia.
How Does Akathisia Differ From Other Forms of EPS?
Akathisia is a type of extrapyramidal symptom.
“The term ‘extrapyramidal’ pertains to the motor system distinct from the corticospinal (pyramidal) tract and is composed of the basal ganglia (caudate, putamen, globus pallidus), subthalamic nucleus, substantia nigra, red nucleus and brain stem reticular formation. The ‘nigrostriatal’ dopamine pathway traverses these anatomical structures and D2 blockade in this region is thought to give rise to EPS. Antipsychotic-induced EPS includes a variety of different iatrogenic movement disorders that can be divided into acute and tardive syndromes. Acute syndromes are those that develop within hours or days of antipsychotic treatment and include acute dystonia, akathisia and parkinsonism. Tardive dyskinesia and tardive dystonia are late onset syndromes that occur after prolonged treatment with antipsychotics. Table I outlines the important characteristics of these EPS variants,” (Pierre 2005).
Table I from (Pierre 2005)
Akathisia and acute dystonia both have a quicker onset (hours to days) compared to the other EPS (weeks to months to years).
Acute dystonia is much easier to treat than akathisia.
Akathisia is the only EPS that causes a subjective feeling of motor restlessness. Dystonia, parkinsonism, tardive dyskinesia and tardive dystonia have different symptoms.
The diagnosis of akathisia is different from other EPS. All EPS (including akathisia) can be diagnosed using the extrapyramidal symptom rating scale.
Akathisia has its own special rating scale called the Barnes Akathisia Rating Scale (BARS).
Tardive dyskinesia also has its own special rating scale called the Abnormal Involuntary Movement Scale.
Simpson-Angus Scale can be used to diagnose dystonia, parkinsonism, and tardive dystonia, but not akathisia and not tardive dyskinesia.
The treatment of akathisia is also different from other forms of EPS. Akathisia is treated with beta blockers and benzodiazepines, whereas dystonia and parkinsonism are treated acutely with anticholinergics and amantadine. Tardive dyskinesia and tardive dystonia are each treated by switching to atypical antipsychotics that are less likely to cause akathisia, such as olanzapine, quetiapine, iloperidone or clozapine.
What is the Mechanism of Akathisia?
The pathophysiology behind akathisia in duloxetine and other SNRIs/SSRIs is through its serotonergic effects on the nigrostriatal pathway which leads to a decrease in dopamine release (Salem et al., 2017).
Neurotransmitter Patterns Associated with Akathisia
What Brain Structures are Involved in Akathisia?
Ventral tegmentum (pars compacta)
Brain stem (locus coeruleus and raphe nuclei)
Basal Ganglia (globus pallidus and putamen)
Accessory motor cortex
How is it Formally Tested?
Akathisia can be formally tested using the Extrapyramidal Symptom Rating Scale (ESRS), developed in 1979, and with the more specific Barnes Akathisia Rating Scale (BARS), developed in 1989.
Akathisia, as well as all of the other EPS, can be assessed using the Extrapyramidal Symptom Rating Scale (ESRS). ESRS is used for epidemiological studies of tardive dyskinesia in patients with schizophrenia using antipsychotic medications (Chouinard 1979). It assesses for symptoms of all of the EPS. The section that assesses akathisia is:
“7. Akathisia
0: Absent
1: Looks restless, nervous, impatient, uncomfortable
2: Needs to move at least one extremity
3: Often needs to move one extremity or to change position
4: Moves one extremity almost constantly if sitting, or stamps feet while standing
5: Unable to sit down for more than a short period of time
6: Moves or walks constantly” (Chouinard 2005).
The ESRS also allows for clinical global impression of severity of akathisia, and asks the clinician, “Considering your clinical experience, how severe is the akathisia at this time?” The clinician is to rate it as “0-absent, 1-borderline, 2-very mild, 3-mild, 4-moderate, 5-moderately severe, 6-marked, 7-severe, 8-extremely severe,” (Chouinard 2005).
The Barnes Akathisia Rating Scale was created in 1989 in order to differentiate akathisia from other movement symptoms (Barnes 1989).
With regards to the objective portion of the scale, a patient’s symptoms would be rated 3 rather than 2 if the patient is unable to sit perfectly still for two minutes. Someone with a score of 3 absolutely cannot sit still.
This scale is a good reminder for clinicians to ask questions surrounding subjective inner restlessness any time a patient starts an antipsychotic or there is a dose increase.
Why is it Underdiagnosed?
“Akathisia is likely to be overlooked or underdiagnosed when both patient and clinician factors are present. Currently, there may be two major problems with underdiagnosis: (1) symptoms that fulfill the diagnostic criteria for akathisia are overlooked, and (2) conditions that do not fulfill the diagnostic criteria but can still benefit from anti-akathisia measures are underdiagnosed,” (Hirose 2003).
Patient factors:
Patient not communicating inner restlessness or atypical subjective expression of “inner restlessness.”
“Initially, most akathisia patients do not voluntarily express feelings of subjective restlessness to clinicians, and some never express it until they are questioned,” (Hirose 2003).
Pseudoakathisia is a term that “was invented to describe a condition in which there are the objective signs of akathisia without the subjective component. Because akathisia occurs commonly in patients with psychiatric disorders such as schizophrenia, it is not clear if pseudoakathisia should actually be considered a ‘pseudo’ form of the disorder, because many patients experience discomfort that they are unable to understand or express clearly,” (Lohr 2015 pg 5-6).
Vulnerable populations such as patients with autism, cognitive dysfunction and/or brain damage may be unable to articulate “inner restlessness.”
Some people may not describe their akathisia experience as “inner restlessness”; many people describe it as anxiety (Hirose 2003).
This can easily be misinterpreted as being related to the primary illness, or side effect of medication, as agitation or anxiety is a common side effect of antipsychotics.
People may also express inner restlessness as “impatience, apprehension, dysphoria, irritation, anger or rage, tension, confusion, fear, vague somatic complaints, and dyspnea, as well as difficulty in concentrating,” (Hirose 2003).
Lack of apparent motor restlessness
“Patients with motor restlessness can voluntarily stay still for a certain period of time, such as during a consultation,” (Hirose 2003).
“Even in severe akathisia, a patient can voluntarily remain still for a certain time when good manners are expected. This suggests that the emergence of motor restlessness depends on the patient’s capacity for patience, which could decrease the objectivity of motor restlessness as a reliable sign,” (Hirose 2003).
Videotaping during relaxed times could compensate for this. Perhaps the patient or their families could record the motor restlessness on their cell phone and show it to the clinician.
Restlessness in body parts other than the legs
“Restlessness in the legs has been considered a common sign highly characteristic of akathisia. Therefore, restlessness referable to the legs has been considered a pathognomonic sign of akathisia, distinguishing it from restlessness due to other etiologies,” (Hirose 2003).
Both the subjective and objective portions of the Barnes Akathisia Rating Scale rely on evaluation of the patient’s legs.
However, Gibb (Gibb and Lees 1986) and Sachdev (Sachdev and Kruk 1994) found leg restlessness in only 27% and 55% of akathisia cases, respectively.
There have been reports that akathisia can occur to varying degrees in other body parts including the head or neck, chest, abdomen, arms (Hirose 2003).
It can, therefore, be overlooked if only restlessness in the legs is considered.
Other prominent psychic symptoms
Exacerbation of hallucinations, anger to the point of violence, mania, disruptive behavior, panic attack, acting out, suicide attempt or suicidal ideation and depression have all been reported as manifestations of akathisia (Hirose 2003).
It is possible that these are not truly manifestations of akathisia, but rather manifestations of the patient’s primary illness, which are more acute, more obvious, and possibly more important to treat than akathisia. It is possible that akathisia may exist even in the setting of an acute illness.
Absence of other extrapyramidal signs
Some studies report akathisia is often accompanied by signs of parkinsonism (i.e., tremor, rigidity, akinesia).
“Consequently, it has been thought that the appearance of parkinsonism following antipsychotic medication might serve as a predictor for the development of acute akathisia” (Hirose 2003).
“The preconception that parkinsonism accompanies akathisia may cause the clinician to fail to consider the possibility of akathisia when parkinsonism is absent in the patient,” (Hirose 2003).
It is important to educate clinicians that akathisia can exist with or without Parkinson’s symptoms. The Barnes Akathisia Rating Scale is a useful tool that assesses akathisia symptoms without the consideration of Parkinson’s symptoms, and, therefore, may help clinicians diagnose akathisia in the absence of Parkinson’s disease.
Clinician factors:
Emphasis on objective restlessness
Akathisia was originally considered to be a fundamentally subjective condition. However, there has been disagreement on the significance of the subjective patient experience versus the clinician’s objective observations (Hirose 2003).
Motor restlessness can be objectively evaluated by the clinician, while it is more challenging to evaluate subjective restlessness. Thus, the clinician tends to favor motor restlessness in their evaluation.
Additionally, subjective restlessness can be considered a nonspecific symptom in psychiatric patients (Hirose 2003).
The Barnes Akathisia Rating Scale incorporates both objective and subjective restlessness, allowing the clinician to equally consider the patient’s subjective experience.
Failure to consider akathisia during antipsychotic therapy
Clinicians should always suspect akathisia in any patient during antipsychotic therapy, as some patients do not spontaneously or voluntarily present typical symptoms of akathisia.
Failure to fully implement anti-akathisia treatments in ambiguous cases
Oral anti akathisia agents such as beta blockers and benzodiazepines have some benefit, but they often do not completely eliminate it. Therefore, failure of patients to respond to these agents does not necessarily rule out akathisia (Hirose 2003).
Strict adherence to research diagnostic criteria
“There is significant variation in clinical features and disagreement as to which are pathognomonic in akathisia that may have led to confusion in diagnosing akathisia,” (Hirose 2003).
The implementation of the Barnes Akathisia Rating Scale has increased diagnostic accuracy.
What are the Consequences of Undiagnosed and Untreated Akathisia?
Untreated akathisia is associated with medication noncompliance as well as violent and self-destructive behavior (Baldassano 1996).
It is distressing to and disruptive for the patient (Baldassano 1996).
Does Akathisia Increase Suicide?
Akathisia, but not other EPS, is associated with suicide.
Risk correlates with severity and duration.
Important to recognize early, as acute akathisia is easier to treat than chronic and tardive akathisia.
Estimates to increase risk by 5% to 10% over baseline (Iqbal, 2007).
Study of 289 patients experiencing first-episode schizophrenia with an 8-week, double-blind trial comparing Haldol and Risperdal found that suicidal ideation was associated with observed akathisia (Seemuller, 2012).
Used Hillside Akathisia Scale
Suicidal ideation was associated with clinician observed akathisia, depressed mood, younger age, and use of propranolol.
18% experienced akathisia
Hansen et al did not find an association between akathisia and suicidality in older patients with treatment-resistant schizophrenia.
Older adults living with schizophrenia may have adapted to psychosis and are possibly less likely to describe suicidality.
The suicide rate among people living with schizophrenia is higher than people living with major depressive disorder. Individuals living with schizophrenia experience incredibly difficult changes in their thinking and perception of the world. Additionally, akathisia is a very distressing sensory motor syndrome that is brought on as a side effect of medication intended to treat psychotic symptoms.
Does Akathisia Increase Homicide Risk?
Case study of 10 patients who attempted or completed suicide and homicide found p450 mutations that might have increased dose and caused akathisia (Lucire 2011).
Issues included 2D6 and nortriptyline, paroxetine, fluoxetine, etc.
The general observation that akathisia can increase violence has been replicated in correlation studies. If someone is prone to aggression and acquires a condition that makes him or her physically miserable, it increases the probability of him or her becoming violent.
Some of these case studies describe situations in which the patient experiences a lack of memory of what happened during these violent crimes. While these cases may seem no different from delirium, it turns out individuals who commit violent crimes may not always remember every detail either.
Anticholinergic medications certainly impair cognition and memory. But if an individual’s affect is brought to the point where he or she becomes overtly violent, this also tends to impair memory function.
Only about a third of people who committed violent crimes can actually give an accurate timeline.
If someone is living with a psychotic disorder and has increased levels of aggression due to akathisia, their memory of violent events may not be crystal clear.
Any Similar or Linked Pathologies?
Restless Leg Syndrome (RLS) has a similar pathology to akathisia (Müller 1994):
Similar to akathisia, as both produce inner tension and anxiety, restlessness, usually in the legs, and the inability to remain still; constant shifting in place and/or pacing.
Akathisia and restless leg syndrome are both associated with iron-deficiency anemia.
Restless leg syndrome can be provoked by antipsychotics (Ekbom 1970).
Table 3 from (Salem et al., 2017)
There is a case report of a patient with Tourette’s syndrome who showed extremely severe akathisia after small doses of antipsychotics (Müller 1994).
SSRI-treated OCD may be a risk factor for antipsychotic induced akathisia.
A study (Ersche 2011) of 22 patients with OCD (20 of whom were on SSRIs) were given a single dose of amisulpride (a selective D2/D3 antagonist).
8 of the 20 SSRI-treated OCD patients described inner restlessness, agitation, feelings of anxiety, irritability, panic attacks, aggression, and satisfied criteria for akathisia.
Two of the eight reported aggravation of OCD symptoms.
“The subjective experience was so distressing that four of the eight affected patients withdrew from the study.”
Adverse reactions emerged at a mean of 6.2 hours (± 2.1 SD) after the amisulpride challenge, and persisted for a mean of 25h (±14.1 SD).
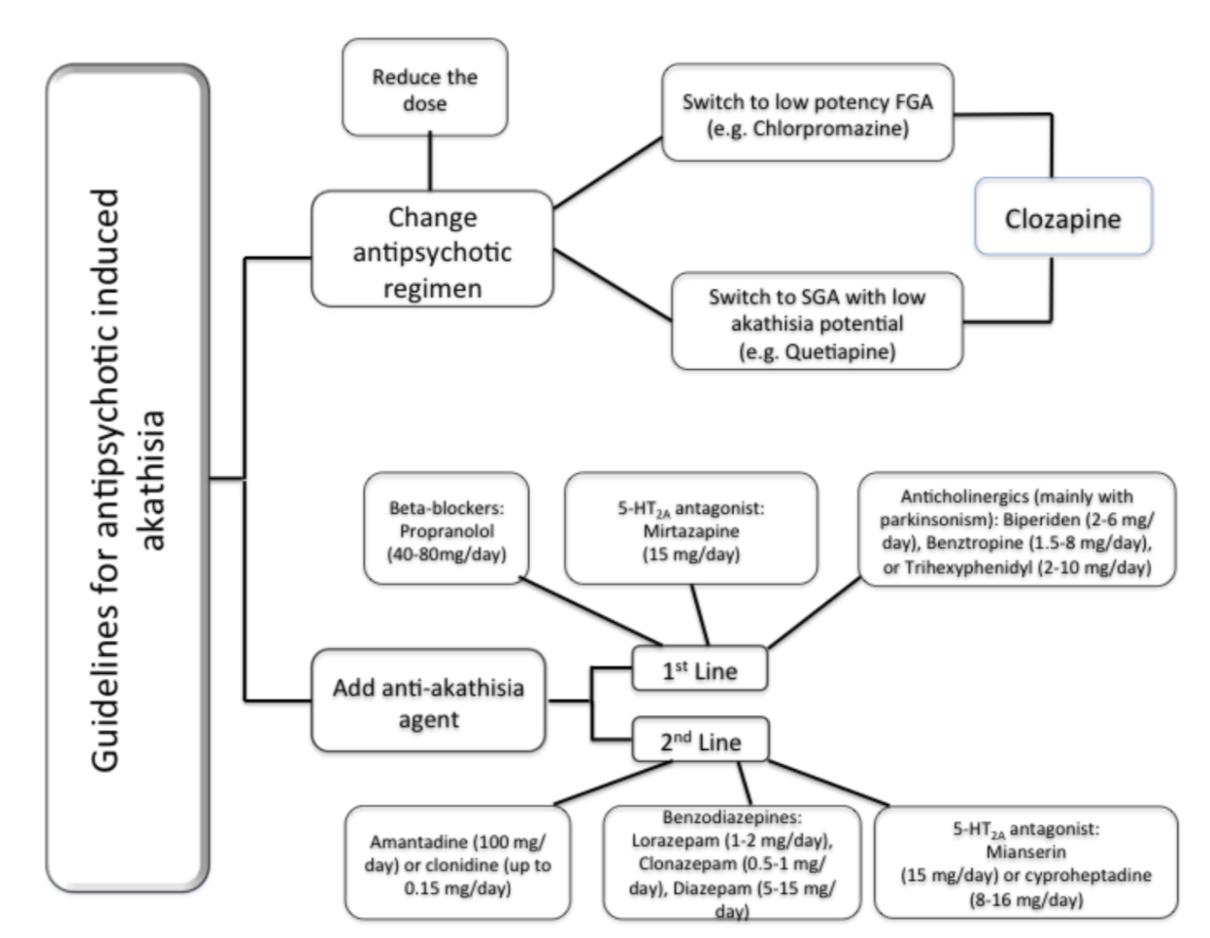
What Treatments are Supported by Evidence?
Figure 1 (Salem et al., 2017).
Initial Interventions (Nonpharmacologic)
Reduce causative agent
Consolidate dosing to bedtime; medication induced akathisia is less likely if peak plasma concentrations occur during sleep. Consolidation to bedtime is less effective for drugs with a longer half-life. I would only do this if the symptoms are mild.
Switch to a medication that has lower risk of causing akathisia (quetiapine, olanzapine, clozapine, iloperidone)
Beta adrenergic antagonists -- must cross blood-brain barrier to be effective
To avoid bradycardia or hypotension, must titrate beta antagonists slowly and monitor pulse and BP.
Propranolol
Propranolol 10mg bid to 30mg TID
Propranolol LA 40mg qd to BID
Metoprolol (if patient has asthma)
Metoprolol 50 to 100mg BID
Metoprolol XL 50mg qd to BID
Benzodiazepines
High-potency benzodiazepines tend to be more effective with less induction of ataxia, impairment of memory consolidation, or excess sedation.
Lorazepam 0.5mg BID to 2mg TID
Clonazepam 0.5mg BID to 2mg TID
Slower onset of action and longer half-life = less prone to abuse, may provide less intraday akathisia variation
Anti-Parkinsonian Medications
These are less effective for akathisia than beta antagonists and benzos, but may be helpful if elements of dystonia or dystonic tremor are also present.
Benztropine 0.5mg BID to 2mg TID
Trihexyphenidyl 2mg BID to 5mg TID
Amantadine 100mg qd to 100mg TID
It is important to address akathisia early. Taking initial nonpharmacologic steps (reduce dose of offending agent, bedtime dosing, or switching to an agent with less dopamine antagonism) is more effective than any of the medications available to treat akathisia.
Efficacy of classic akathisia medications
Acute akathisia: 30% resolution, 40% improved, 30% little benefit
Subacute akathisia: 10% resolution, 30% improved, 60% little benefit
Tardive akathisia: <5% resolution, 20% improved, 75% little benefit
Emerging Medications for Akathisia
Mirtazapine
In a 5 day study (N=26), mirtazapine 15mg q bedtime had a higher response rate (53.8%) than placebo (7.7%) (Puyurovsky 2003).
In a 7 day study (N=90), mirtazapine 15mg q bedtime had higher response rate (43.3%) than propranolol 80mg qday (30.0%), and both were higher than placebo (6.7%) (Puyurovsky 2006).
N-acetyl cysteine (NAC)
In a 4 week study (N=140 patients with schizophrenia, 84 completers) of 1000mg BID NAC vs placebo, there was moderate reduction of akathisia (Berk 2008)
For patients who are prone to developing akathisia, NAC might be protective when starting an antipsychotic
Pyridoxine (vitamin B6)
In a 5-day study (N=20), compared to placebo, B6 300mg BID resulted in a downward trend in objective rating, decreased awareness of akathisia, and decreased distress due to akathisia (Lerner 2004).
For patients who are prone to developing akathisia, vitamin B6 might be protective when starting an antipsychotic.
Mianserin (tetracyclic antidepressant used in Europe, not available in the U.S.)
In a 5-day study (N=60), mianserin and B6 did not differ in terms of improvement of akathisia symptoms (Miodownik 2006).
Summary
Catch akathisia early! The faster you identify and treat it, the better the outcome. Chronic and tardive akathisia are very difficult to treat, and may persist for decades or a lifetime.
When starting any medication, make sure your patient knows that he or she can reach out to you if he or she begins to experience adverse reactions.
When starting an antipsychotic (or any medication that could potentially cause akathisia), tell your patient to reach out right away if he or she experiences restlessness.
When following up with patients who started a new medication, always screen for restlessness. At least ask, “Have you felt restless?” If they answer yes, ask “Do you feel like you need to walk around more?” If they answer yes, consider utilizing the Barnes Akathisia Scale to determine the severity of the presentation.
Next, consider the timeline to identify which type of akathisia, and to understand what is the offending agent.
Consider the patient’s medication list. Had the patient been on a medication that inhibits the metabolism of the newly introduced drug?
Consider obtaining plasma concentration of the offending agent, as you may see a much higher plasma concentration than you would expect if a patient is on a small dose. Remember, some people are poor metabolizers and you may see adverse reactions at even a very small dose. Dose is a poor guide to adequacy of treatment and potential of adverse reactions.
Take action immediately! Do not wait for the result of the plasma concentration.
After that, you can manage it. If the offending agent is risperidone, consider switching to an antipsychotic with less akathisia potential (olanzapine or quetiapine). If you cannot switch antipsychotics, consider decreasing the dose of the offending agent. You could also consider adding a beta blocker or benzodiazepine.
Follow up with your patients with akathisia frequently! See them multiple times per week to see if there is improvement.
If you suspect akathisia, speak up and communicate this with your team! Print out the Barnes Akathisia Scale and circle what you see and have a discussion with your team.
Akathisia presents in many different ways, and if you are able to recognize and treat it, your patients will have a much better quality of life.
Sources:
Abbott, J. H. (1991). In the belly of the beast: Letters from prison. Vintage.
Asser A, Taba P. Psychostimulants and movement disorders. Front Neurol. 2015; 6: 75.
Duma SR, Fung VS. Drug-induced movement disorders. Aust Prescr. 2019;42(2):56-61.
EKBOM. Restless legs. In: VINKENPJ, BRUYNGW, eds. Handbook of clinical Neurology, Vol. 8: Amsterdam: North Holland. 1970: 31 1-320.
Mohr P, et al. Ladislav Haskovec and Akathisia 100th Anniversary. Br J Psychiatry 2002; 181: 537
Riesselman A, El-Mallakh RS. Akathisia with azithromycin. Ann Pharmacother. 2015; 49(5): 609.