Episode 145: How to Manage Aggression with Psychopharmacology in an Inpatient Setting
By listening to this episode, you can earn 1 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: David Kim, Garrett Rossi MD, Michael Cummings MD, David Puder MD
Dr. Puder and Dr. Cummings have no conflicts of interest to report for the audio that goes with this article.
Overview & Epidemiology
Violence and aggression are often used interchangeably, with subtle distinctions differentiating the two. Aggression is an umbrella term that encompasses violence and is defined as actions that lead to harm towards self, others, or objects, while violence is defined as actions that lead to harm, specifically toward other individuals (Newman, 2012). Aggression, according to the 3-factor approach initially detailed by investigators from the New York State Hospital system, is categorized into three types of assault: impulsive, predatory/organized, and psychotic. Impulsive aggression was the most common type at 54%, with predatory/organized type (29%) and psychotic type (17%) trailing behind (Quanbeck CD, 2007; Meyer et al., 2016). This episode aims to explore the management of agitation, aggression, and violence in the inpatient setting.
Schizophrenia and Comorbid Substance Use
A wide array of evidence has historically supported the uncontroversial correlation between violence and mental health disorders, particularly schizophrenia and substance use. A random community sample of 10,000 people reported that in a 12-month span, self-reported violence increased by up to fivefold in patients with schizophrenia compared to their counterparts with no schizophrenia. Furthermore, violence rates increased in individuals with a diagnosis of alcohol use and drug use compared to those with no diagnosis. Violence rates for the former increased by up to 12 times while rates for the latter increased up to 16 times (Maden, 2004). Further research into pharmacological treatments for violence and aggression has proven difficult. Due to the nature of a violent individual, he or she is less likely to commit and remain in the study or consent for treatment (Fazel et al., 2014).
Multiple studies have reproduced the association between schizophrenia and increased risk of violence (Fazel et al., 2009; Iozzino, 2015) especially in those having a first episode and those with co-morbid substance use. In the early 1990’s, Swanson published a revolutionary epidemiological study showing a 6.3% difference (8.4% in schizophrenia vs 2.1% in control group) in one year prevalence of violent behavior (Swanson, 1994). Studies since have exhibited similar findings. Meta-analysis of 35 studies involving 23,972 inpatients from acute psychiatric wards showed that 17% of patients would perpetrate a violent act during their inpatient stay (Iozzino, 2015). This study further reported that higher inpatient violence rates were seen with patient characteristics of male gender, involuntary hospitalization, and substance use.
Comorbid severe mental health illness with substance use disorders have a synergistic effect on violence risk. A systematic review of 20 studies comprised of 18,423 individuals with schizophrenia and other psychotic disorders yielded a prevalence rate of 8.9 (5.4-14.7) in psychosis with comorbid substance use and a prevalence rate of 2.1 (1.7-2.7) in psychosis without the comorbidity (Fazel et al., 2009). Surprisingly, the risk of violence in those with psychosis with substance use comorbidity were comparable to those with substance use disorder without psychosis, suggesting that substance use and psychotic symptoms are independent risk factors for violence.
Of note, other factors for violence risk consist of past and present experiences. A history of childhood trauma and neglect, growing up around antisocial behavior, binge drinking, and stressing excessively were associated with increased violence risk (Van Dorn et al., 2012).
Bipolar Disorder
While aggression is not a formal symptom according to DSM-5 criteria for bipolar disorder, aggression is commonly seen in manic and mixed episodes of bipolar disorder. A factor analysis showed that triggers for aggression were paranoia and irritability (Cassidy et al., 1998a) with similar frequencies of aggression between the two subtypes of bipolar disorder (Cassidy et al., 2000).
Lifetime prevalence of aggressive behaviors in bipolar disorder and schizophrenia are comparable to one another. Several large scale epidemiological studies have been conducted to examine the prevalence of bipolar disorder. A sample representing the population of the United States collected by the National Comorbidity Survey (NCS) between 1990 and 1992 found aggressive behavior in 12.2% of individuals with a diagnosis of bipolar disorder (Corrigan & Watson, 2005). The NESARC study from 2001-2002 assessed the prevalence of aggressive behavior in 43,093 adults representing the general U.S. population. It revealed a lifetime prevalence of aggressive behavior in those with bipolar I and bipolar II to be 25.34% and 13.58%, compared to 0.66% in persons without a lifetime psychiatric disorder (Latalova, 2009).
Like schizophrenia, the presence of comorbidities, specifically alcohol/substance use disorder and personality disorders, increase the risk of violence in bipolar disorder. The prevalence of aggressive behavior in pure bipolar disorder (without comorbidity) was found to be 5.12% and 2.52%, compared to pure alcohol dependence and drug dependence which was 7.22% and 11.32%, respectively (Pulay et al., 2008). Violence risk was the highest in bipolar patients with comorbid substance use (adjusted odds ratio, 19.9; 95% CI, 14.7-26.9), while bipolar patients had elevated risk for violence regardless of comorbid substance use (adjusted odds ratio, 3.1; 95% CI, 2.6-3.8) (Fazel et al., 2010a).
Pathophysiology of Aggression
Historically, confinement was the primary intervention for aggressive behaviors in psychopaths, rather than using pharmacologic therapies. Currently, pharmacologic therapeutic interventions are being used ranging from D2 antagonism to clozapine. Studies have examined epigenetics, environmental factors, and also the interaction between the two, as possible pathways that contribute to the development of psychopathic personality traits. A meta-analysis of 20 male cohorts investigated the interaction between childhood adversity with the regulation of monoamine oxidase, type A (MAOA). The study showed that childhood adversity or maltreatment correlated with decreased transcription efficiency of MAOA. The attenuated levels of MAOA lead to diminished norepinephrine and serotonin availability, ultimately leading to the development of antisocial traits (Byrd & Manuck, 2014; Haller et al., 1998). Moreover, another potential cause of serotonin hypofunction is due to the polymorphisms of serotonin transporter genes with subsequent decreased levels of synaptic serotonin (Pavlov et al., 2012). Of note, schizophrenic patients are genetically predisposed to monoamine oxidase dysfunction (Hoptman, 2015).
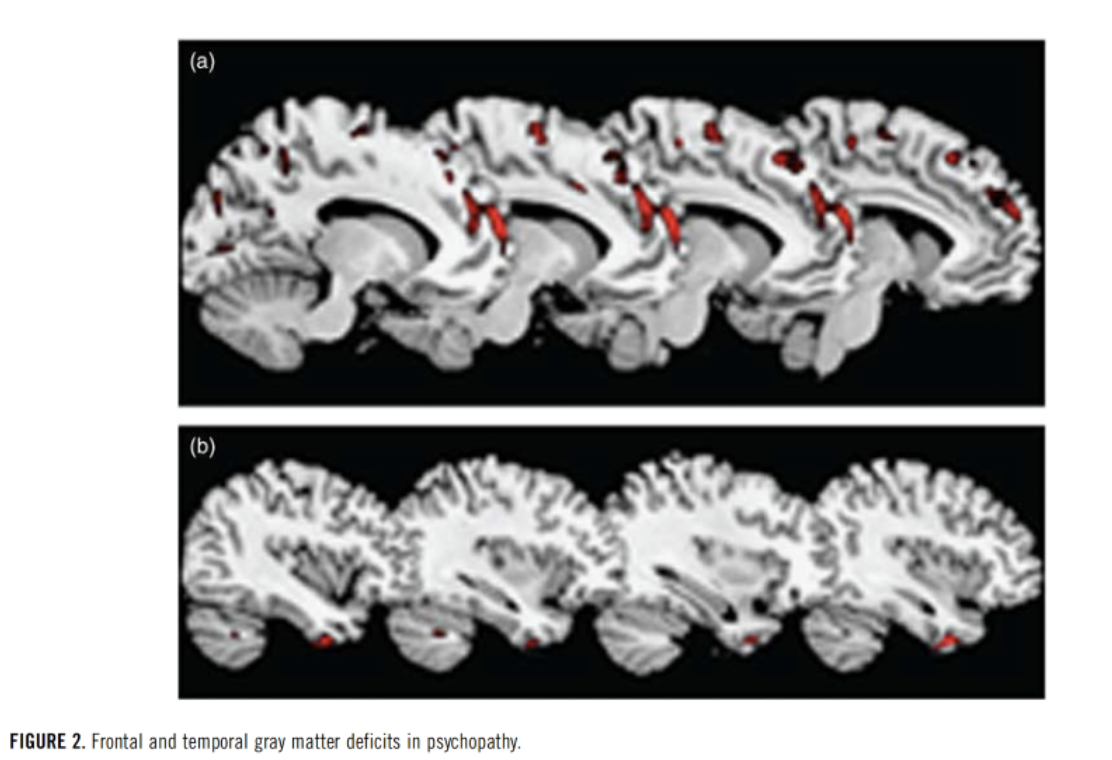
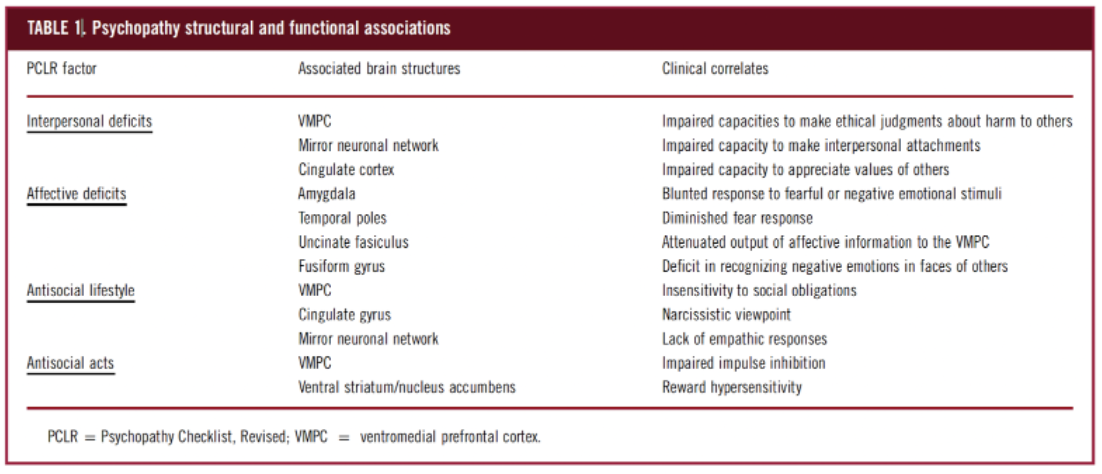
Decreased gray matter in numerous paralimbic and limbic areas, namely the amygdala and the ventromedial prefrontal cortex (VMPC), have been implicated as areas of research for treatment in improving psychopathic tendencies (Cummings, 2015; Ermer et al., 2012).
The amygdala and its associated structures are well-known regulators of the fear and anxiety response, while the VMPC processes provoke stimuli and determine a person’s response. The amygdala and VMPC collectively function as a top-down inhibitory system and have been hypothesized to underlie the impulsive aggression seen in psychopaths (Cummings, 2015; Meyer et al., 2016). MRI studies have shown that attenuated neural connectivity of the amygdala and the ventromedial prefrontal cortex (VMPC) via the uncinate gyrus was found in psychopaths (Contreras-Rodriguez et al., 2014). Furthermore, the 3 distinct types of aggression may have common affected neuroanatomical pathways. A lesion in the VMPC leads to impulsive aggressive responses by failing to make appropriate risk/reward assessments that normally inhibit aggressive responses (Meyer et al., 2016).
Etiologies of Aggression
Derived from the California State Hospital Violence Assessment and Treatment (Cal-VAT) guidelines (Stahl et al., 2014)
Psychotic aggression (Volavka & Citrome, 2011)
Misunderstanding or misinterpreting external stimuli
Positive symptoms of psychosis
Paranoid delusions
Command Hallucinations
Grandiosity
Autonomic Arousal
Impulsive aggression (Volavka & Citrome, 2011)
Hyperreactivity to stimuli
Emotional hypersensitivity
Exaggerated threat perception
No planning or organization
Autonomic arousal
Predatory aggression (Hare & Neumann, 2008)
Planned violence
Goal-directed
Lack of remorse
Autonomic arousal absent
Physical conditions contributing to violence risk (Hankin et al., 2011)
Psychomotor agitation
Akathisia
Pain or physical discomfort
Delirium
Intoxication or withdrawal
Complex partial seizures
Sleep issues
Abnormal laboratory results that may contribute to violence risk (Joshi et al., 2012)
Plasma glucose
Plasma calcium
WBC count to rule out sepsis
Infectious disease
Hyponatremia or hypernatremia
Oxygen desaturation
Serum ammonia
Thyroid status
Sedimentation rate if history of inflammatory disease
Antipsychotics Overview
Dopamine Antagonists and Atypical Antipsychotics
Positive psychotic symptoms such as hallucinations and delusions generally prompt patients to behave in ways that may lead to aggression and, at worst, criminal offenses, while negative symptoms affect psychosocial and environmental processing. Mainstay treatment for these positive symptoms has been dopamine D2 receptor antagonism in the mesolimbic tract, whereas treatment for negative symptoms has been shown to be more effectively treated with dopamine partial agonist antipsychotics acting on the mesocortical pathway (Veerman et al., 2017; Stahl, 2017). The dopamine D2 receptor antagonist antipsychotics deploy a shared fundamental mechanism of action: inducing the depolarization blockade of the dopamine neurons in the mesolimbic dopamine pathway (Grace, 1992).
Atypical antipsychotics have decreased binding to dopamine D2 receptors while having increased affinities for norepinephrine(α1 and α2) and serotonin (5-hydroxytryptamine1A, 2A, 2C, 3, 6, 7) receptors (Miyamoto et al., 2005) among other receptors. While clozapine and olanzapine are firmly established as effective interventions for acute agitation in schizophrenic patients, short acting IM second-generation antipsychotics such as ziprasidone, olanzapine, and aripiprazole, yielded effect sizes comparable to those of haloperidol and lorazepam (Citrome, 2007). Atypical antipsychotics are generally more favorable due to their decreased tendencies to cause dystonia and Parkinsonism. Of note, clozapine’s mechanism of action is unique, as its antipsychotic effects are more likely due to glutamate modulation rather than its effects on the mesolimbic tract (Cummings et al., 2019).
Antipsychotic Trials
An appropriate antipsychotic trial in duration and dosage was deemed to be a trial of at least 6 weeks and a 600 mg minimum equivalent of chlorpromazine. Four weeks was deemed sufficient for long-acting injectables (Cummings et al., 2019). Medications must be titrated until the patient responds clinically or develops intolerance to medications. High dosages or plasma concentrations of medication are not sufficient reasons for terminating treatments during trials. If a violent schizophrenic patient does not respond clinically to moderate doses of D2 antagonists, tolerates medications without extrapyramidal side effects (EPS) or akathisia, or refuse/fails a clozapine trial, these patients may be candidates for high plasma-level antipsychotic therapy (Meyer, 2014). A minority of schizophrenic patients display immense tolerance for D2 antagonism without developing EPS or akathisia.
Consequences of Antipsychotic Nonadherence
A modifiable risk factor for violent behavior in psychotic patients is antipsychotic nonadherence. Rates of non-adherence for schizophrenic patients were reported to be from 40% to 50%, while all hospitalizations due to non-adherence were reported to be 50% to 55% (Mohr & Volavka, 2012). A prospective observational study with a 3-year follow-up, showed that antipsychotic non-adherence in psychotic patients led to higher number of rehospitalizations, placing financial burdens on institutions and requiring more staff for patient care. In particular, these patients exhibited behavioral dysfunction by using substances at an increased rate in the following two years, being 2.2 times more likely to be arrested (OR = 2.22 [1.53-3.24]) and having 1.8 times more likely chance of falling victim to criminal offenses (OR = 1.82, [1.42-2.34])(Ascher-Svanum et al., 2006). Conversely, psychiatric patients who were adherent to their antipsychotics were found by a Swedish population study involving 82,647 individuals to exhibit a 45% reduction in violent crimes (hazard ratio [HR] 0.55; 95% CI 0.47-0.64) compared to their non-adherent counterparts (Fazel et al., 2014), suggesting that medication adherence is crucial to behavioral regulation.
Treatment Resistance and Pharmacokinetics of Antipsychotics
There are two principle routes for treatment failure with dopamine antagonists. The first is treatment resistance, indicated by the patient’s inadequate response of reduction of psychotic symptoms of 20% to 30% with two sufficient dopamine antagonist trials. Treatment resistance predicts a subsequent, poor response to further antipsychotics (Volavka, 2013; Cummings et al., 2019). The second route for treatment failure is due to pharmacokinetics.
Generally, therapeutic D2 receptor blockade has been hypothesized to be around 80%. Higher than the 80% receptor blockade is not helpful as the D2 receptor curves plateau past this percentage. This means that at greater than 80% receptor blockade, higher doses result in minimal increases in D2 receptor blockade and increase the risk for side effects (Uchida et al., 2011). An example given by the study (Uchida et al., 2011) showed that tripling the dosage of antipsychotic only increased the receptor blockade by approximately 3% (80% to 83%). Of note, a study involving 28 antipsychotic-naive schizophrenic patients showed that the optimal response occurs with 60% to 80% D2 blockade (Sanne Wulff et al., 2015).
Plasma antipsychotic levels, rather than dosage, is a more accurate measure when investigating antipsychotic efficacy. Plasma levels are more reliable measures of receptor occupancy than the administered dose (Urban & Cubala, 2017). Advantages to measuring antipsychotic plasma concentrations include assessing metabolism, determining optimal plasma concentrations, and investigating possible causes of decompensation or worsening side effects (Dahl, 1986). Differences in clinical response based on antipsychotic plasma concentrations and dose are due to various factors. These factors include adherence, absorption, distribution, catabolism, and elimination (Fan & de Lannoy, 2013). This is the result of extensive first-pass metabolism by the cytochrome P450 system, and the high affinity of antipsychotics for P-glycoprotein (PGP), an efflux transporter (Meyer, 2007). Simultaneous use of antipsychotics and other CYP450 inducers such as cigarette smoke, carbamazepine, rifampin, and phenytoin, among others, can impact antipsychotic plasma levels. Higher doses may be required for those using a CYP450 inducer concomitantly with an antipsychotic. Switching to a medication that does not induce CYP enzymes such as lithium is one strategy to avoid this problem (Meyer, 2007).
If a schizophrenic patient displays persistent violent behavior despite an adequate trial of antipsychotic medication, a lack of EPS symptoms or akathisia, plasma antipsychotic levels can play a crucial role in assessing the reason for persistent violence. Schizophrenic patients with persistent violent behavior despite these measures should be treated with clozapine and in the case of refusal or contraindications, olanzapine is an alternative option (Meyer, 2014).
Treatment/Management of Agitation in an Inpatient Setting
Overview
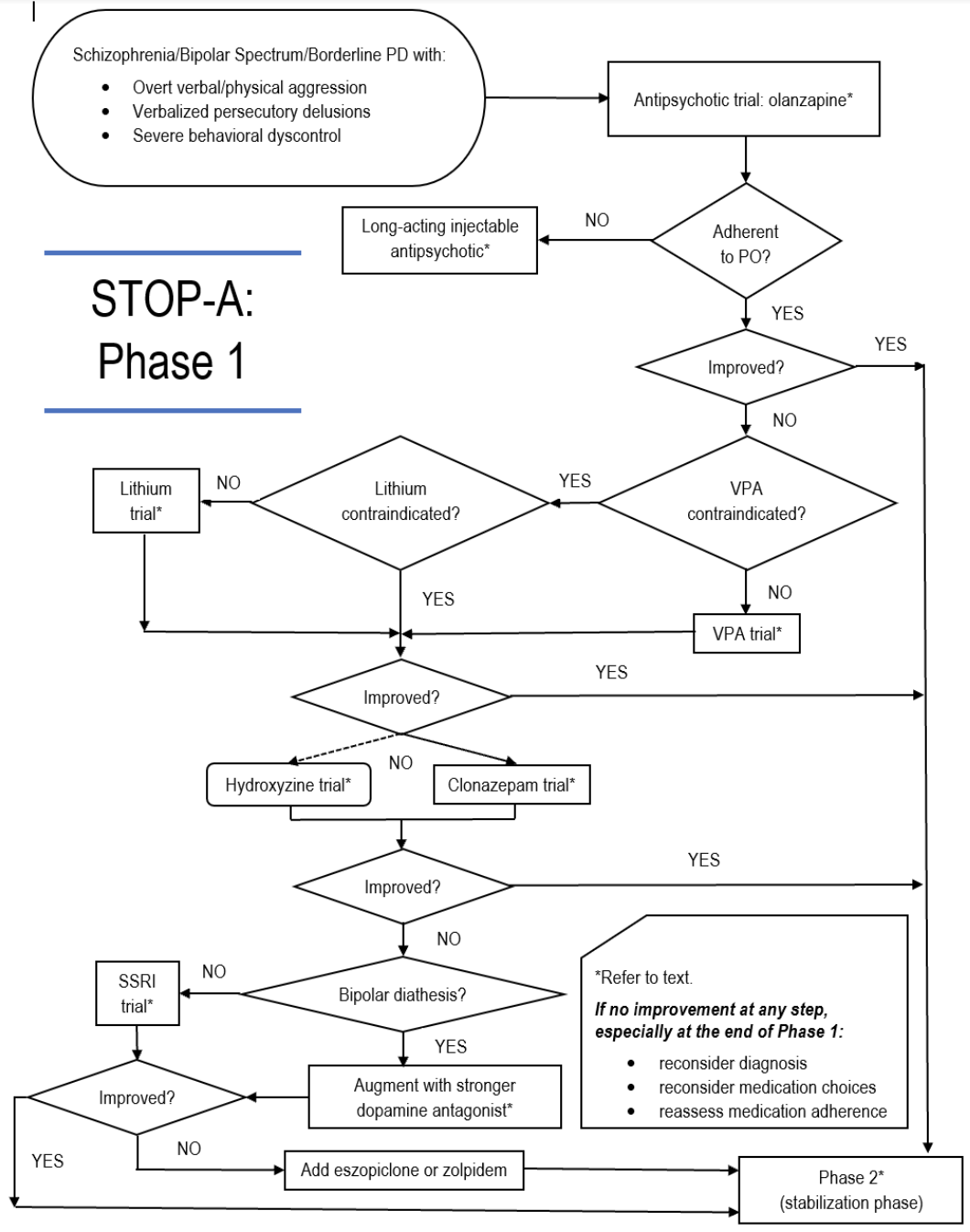
There are three phases to the treatment of agitation in an inpatient setting. The first phase involves decreasing acute agitation and risk of violence to permit further evaluation. The second phase is to treat the underlying etiology of agitation to stabilize the patient enough to add adjunctive therapies such as rehabilitation or other psychotherapeutic interventions. The third phase is to continue stabilization while encouraging and promoting normative socialization. This episode aims to explore an algorithm from the book, Management of Complex Treatment-resistant Psychotic Disorders.
An acutely aggressive or violent patient can have vastly different presentations. The provider should begin by assessing if the patient is capable of being interviewed, and also formulating the underlying etiology of agitation (psychosis vs bipolar vs drugs vs alcohol withdrawal vs delirium). The distinctive principle in treatment is being able to reconsider diagnostic formulations. If treatment is not yielding an adequate therapeutic response, question the hypothesized etiology and reassess the patient.
Initial treatment for patients acutely agitated with risk of hurting self/others
Providers can use interventions classically used for acute psychomotor agitation: fluphenazine 5 mg or haldol 5 mg, with lorazepam 1 mg, with hydroxyzine 25 mg IM. Hydroxyzine is recommended over benztropine and diphenhydramine, as it is a 1st generation antihistamine that readily passes the blood-brain barrier without causing anticholinergic symptoms (impaired memory and cognition, dental caries, urinary retention, etc.), and possible anticholinergic delirium. Rather than ordering a single high dose of the medications, the prescription recommendation is to use lower doses at higher frequencies to titrate plasma levels of medications to therapeutic levels without relapse of symptoms. Medication administration should not exceed 6 doses in a 24 hour period.
Treatment of the underlying disorder
Patients continuing to show persistent agitation should be treated for the underlying cause of the acute agitation. Olanzapine is the initial treatment due to its anticholinergic, dopamine antagonizing, and glutamate-modulating properties (at high plasma concentrations), along with its known antipsychotic and mood stabilizing properties. Olanzapine reaches peak plasma concentration after 6 to 9 hours. Of note, do not use fluvoxamine and olanzapine as fluvoxamine inhibits the P450 1A2 system, increasing risk for olanzapine toxicity.
If there is no therapeutic response, a trial of divalproex is recommended over lithium due to divalproex’s wider therapeutic index. Lithium may be used over depakote if patient has bipolar illness or if the patient is prone to bipolar diathesis. Lithium, with its neurotrophic properties, can be helpful in treating acute agitation if the etiology of the agitation is due to manic or depressive episodes.
Patients exhibiting symptoms of agitation, aggression, or violence should always be assessed for lab abnormalities as the underlying etiology. Common lab values to check are plasma ammonia if the patient exhibits signs and symptoms of delirium (especially if using divalproex), glucose, calcium, sodium, sed rate.
Patients with drug intoxication or withdrawal, specifically methamphetamines, require antipsychotics, benzodiazepines, and at times divalproex until drugs wear off. Alcohol withdrawal requires a cross tapering with a drug that will replace alcohol (e.g., lorazepam) to prevent delirium tremens, with benzodiazepines and magnesium sulfate to decrease seizure risk and blood pressure control.
Delirium traditionally has “waxing and waning” levels of alertness and is a common cause of agitation in the inpatient setting. Delirious patients present with alternating agitation and stupor. The human body is known to have various compensatory mechanisms to preserve the brain. As a result, the presence of delirium reflects a state of the brain where the protective measures of the brain have failed. It has been hypothesized that peripheral infections and inflammatory processes cause excessive release of cytokines IL-6 and IL-8, damaging astrocytic glial cells. Dysfunction of astrocytes causes impairment of the brain’s ability to wash out toxic molecules and modulate CSF production, maintenance, and metabolism, ultimately leading to suboptimal levels of arousal. Treatment of the underlying cause of delirium is recommended when delirium is suspected to be the cause of acute agitation.
Persistent agitation despite attempting to treat underlying cause
At this point, reconsider diagnostic formulations, but also ask two questions: (1) is the patient taking their medications?; and (2) does the patient have abnormal metabolism of the drugs?
For ongoing psychomotor agitation, patients should be considered for sedation for acute management using hydroxyzine, a first generation antihistamine, or clonazepam, a benzodiazepine that increases GABAergic transduction signaling. Hydroxyzine can be used as an anxiolytic and sedative and the recommended dose is 25 mg to 100 mg PO or IM BID. Clonazepam is indicated if valproic acid or lithium is contraindicated. Recommendation for the initial dose is 1 mg TID or QID with adjustments to 0.5 mg to 2 mg TID or QID. These medications are used only in the acute setting and should be discontinued with resolution of symptoms or resistance to treatment.
If a patient is exhibiting symptoms refractory to hydroxyzine or clonazepam, consider bipolar diathesis as a cause. If a patient is inclined for bipolar diathesis, augment with a stronger dopamine antagonist. With low suspicion for bipolar diathesis, consider initiating an SSRI trial. SSRIs are indicated for persistent aggression or dementia related aggression. SSRI’s exert effect by increasing serotonin bioavailability in the limbic system, leading to decreased irritability and impulsivity. Recommendation is to dose daily and if terminating medication to taper over two to four weeks to avoid withdrawals. Contraindications include bipolar patients and autism spectrum disorder due to the risk for increased irritability and aggression.
As sleep deprivation from disrupted or inefficient sleep can be a cause for agitation in hypomanic and manic individuals; inducing sleep is an effective mode of treatment of the underlying cause of agitation. A recommended dose in this setting is zolpidem 10 mg qhs. While effective, zolpidem has a relatively short half-life. In this circumstance, eszopiclone, a benzodiazepine receptor agonist, should be initiated starting at 4 mg qhs.
Clozapine
Clozapine is viewed as the gold standard therapeutic intervention for violent patients with schizophrenia (Cummings et al., 2019; Frogley et al., 2012; Topiwala & Fazel 2011). Clozapine is a dopamine (D4 > D2) and serotonin agonist, partial 5-H1TA, and a muscarinic (M1-M5), histamine, and alpha-1 adrenergic antagonist (Haidary & Padhy, 2021). Clozapine has also been hypothesized as having glutamate-modulating properties, which may explain its superiority over other antipsychotics (Veerman et al., 2014). The FDA approved maximum dose is 900 mg per day (Haidary & Padhy, 2021). Clozapine does not usually exert its anti-aggressive effects until an estimated dose of 500 mg (Volavka, 2013). Indications for clozapine include patients with schizophrenia who failed 2 or more antipsychotic trials of adequate dose and duration (Krakowski, 2006).
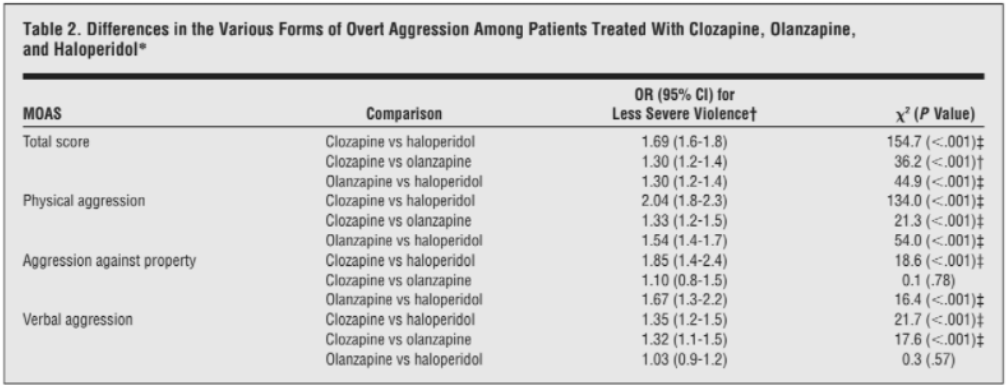
A review of the literature revealed evidence for the anti-aggression effects of clozapine independent of its antipsychotic effects (Frogley et al., 2012; Meyer 2014). A randomized, double-blind trial consisting of 157 schizophrenic individuals treated with either clozapine, olanzapine, risperidone, or haloperidol, demonstrated that clozapine had higher efficacy than risperidone and haloperidol, and similar efficacy to olanzapine in the Positive and Negative Syndrome Scale (PANSS) (Volavka et al., 2002). A second randomized, double-blind, parallel-group trial of 12 weeks involved 110 aggressive patients with schizophrenia spectrum disorders treated with either clozapine, olanzapine, or haloperidol. Clozapine exhibited higher efficacy than olanzapine and haloperidol in two areas: reduction of physical aggression, determined by the Modified Overt Aggression Scale (MOAS) physical aggression score, and overall aggression reduction, determined by the MOAS total score. Olanzapine demonstrated greater efficacy in relation to haloperidol. Confirming the established parameter that clozapine’s anti-aggression effects are independent of its antipsychotic properties, this trial also demonstrated that clozapine did not differ significantly from olanzapine or haloperidol in reducing psychiatric symptoms based on the PANSS total score and the PANSS subscales (Krakowski et al., 2006).
Clozapine, however, is not a definitive treatment for aggression. In one study, up to 50% of patients did not respond to clozapine (Lieberman et al., 1994). Another study from the UK found that patients responded to clozapine at rates from 50% to 60% (Citrome, 2013). Of note, clozapine’s therapeutic effects decline after 2.8 years of treatment in schizophrenic patients refractory to treatment (Cummings et al., 2019). When patients do not respond to maximum plasma levels of clozapine with adjunctive D2 antagonism, the next step is to offer valproic acid, SSRIs, and β-adrenergic antagonists (Goedhard et al., 2006; Citrome and Volavka, 2011).
Olanzapine (Zyprexa)
Olanzapine is a well-known therapeutic agent for acute agitation. It’s a D2 antagonist with low affinity for the receptors and also a 5HT2A receptor antagonist in the frontal cortex (Thomas & Saadabadi, 2022). Olanzapine is relatively effective in treating physical aggression compared to haloperidol in violent schizophrenic individuals and has an odds ratio (OR - 1.3(1.2-1.4) for reduced violence. This odds ratio was comparable to the odds ratio (OR - 1.3(1.2-1.4) comparing clozapine and olanzapine (Krakowski et al., 2006). The OR for a double-blind trial consisting of 1445 individuals with schizophrenia resulted in olanzapine having similar effects on anti-aggression with other atypical antipsychotics, not including clozapine (Swanson et al., 2008a). However, in an earlier study comparing olanzapine and risperidone in a 1-year trial, olanzapine reduced violence risk significantly while risperidone failed to yield significant reduction of violence risk (Swanson et al., 2004). Recommended initial doses of olanzapine are 10 mg PO to 20 mg PO. If a patient refuses or is intolerant of oral medications, a 10 mg IM dose can be given (Stahl et al., 2014). Compared to the 50% to 60% response rate to clozapine, the response rate to olanzapine of schizophrenic patients refractory to treatment using Kane’s criteria was only 10% (Conley et al., 2003).
Risperidone (Risperdal)
In open-label studies of risperidone for agitation in schizophrenic patients (Chengappa et al., 2000, Bitter et al., 2005) and one double-blind study involving 139 randomized patients (Czobor et al., 1995), risperidone was found to reduce agitation and hostility compared to placebo. However, when compared to other antipsychotics, use of risperidone did not yield significant results (Swanson et al., 2008a). A unique indication for risperidone is failure of treatment response to olanzapine 20 mg BID for acute agitation. If the patient fails to respond by the end of one week with olanzapine, clinicians should add risperidone 2 mg QHS, increasing dose by 2 mg every other day to a target dose of 6 mg QHS (Cummings & Stahl, 2021).
Valproic acid (divalproex) & Lithium
Both lithium and valproic acid, which are commonly used as mood-stabilizing agents in bipolar disorder, can be used as adjunctive treatment for aggression. They both act as agents to minimize limbic activity at the amygdala (Citrome & Volavka, 2011; Correll et al., 2017). Both should be loaded with the goal of achieving a high but safe plasma concentration and continued at this level for no more than 8 weeks (Cummings & Stahl, 2021).
VPA is a possible next step in the management of patients displaying impulsive aggression who did not respond clinically to maximum plasma concentrations of clozapine with adjunct D2 antagonism (Goedhard et al., 2006; Citrome and Volavka, 2011). VPA should be titrated to the optimal plasma concentration of 100 to 120 mcg/ml and maintained at a range of 80-120 mcg/ml (Cummings & Stahl, 2021). Furthermore, VPA should be dosed twice per day to minimize seizure risk. Of note, VPA is associated with decreasing plasma concentration of atypical antipsychotics, olanzapine (Haslemo et al., 2012) and clozapine (Longo & Salzman, 1995), leading to subtherapeutic levels of the antipsychotics.
Lithium has anti-aggression properties in patients with intermittent explosive disorder (Jones et al., 2011), but its effects on agitation in the schizophrenic population are inconclusive. The initial dose of lithium should be 600 mg nightly, increasing the dose by 300 mg every other day to a maximum nightly dose of 1200 mg. Contrary to other medications mentioned, plasma concentrations are not the most useful indicators in predicting clinical response. The minimum plasma concentration for therapeutic response is 0.6 meq/L (Cummings & Stahl, 2021). Use of lithium calls for strict monitoring due to its vast side effect profile (polyuria, tremor, cognitive environment) and requires immediate cessation with either intolerance or lack of clinical response (Citrome 2009).
Long Acting Injectables (LAI)
As mentioned above, non-adherence is a modifiable risk factor for violence risk. Non-adherence leads to relapse, rehospitalizations, and higher probability of engaging in violent behaviors. Long acting injectables (LAI), also known as depot injections, are effective in promoting medication adherence so as to minimize the consequences of non-adherence (Haddad et al., 2014; Mohr & Volavka, 2012). A narrative study comprising cross-sectional, retrospective, and prospective studies with case studies and randomized trials, explored the benefits of LAI to treat violence and aggression in psychotic patients (Mohr et al., 2017). As relapse of symptoms can occur following cessation of medication, LAIs reduce chance of relapse due to prolonged plasma concentrations (Mohr et al., 2017; Cummings et al., 2019). Due to a one-time dose of high levels of antipsychotics, LAI medication cannot be titrated to therapeutic levels. There is also the possibility of prolonged side effects compared to oral antipsychotics (Mohr et al., 2017). While there are no controlled trials to confirm if LAIs reduce the risk of violence, retrospective studies have confirmed that LAIs were more effective than their oral counterparts at reducing severity of symptoms including aggression, hostility, violent acts, and criminal offenses (Mohr et al., 2017).
References:
Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006 Mar;67(3):453-60. doi: 10.4088/jcp.v67n0317. PMID: 16649833. (n.d.).
Bitter I, Czobor P, Dossenbach M, Volavka J. Effectiveness of clozapine, olanzapine, quetiapine, risperidone, and haloperidol monotherapy in reducing hostile and aggressive behavior in outpatients treated for schizophrenia: a prospective naturalistic study (IC-SOHO). Eur Psychiatry. 2005 Aug;20(5-6):403-8. doi: 10.1016/j.eurpsy.2005.01.009. PMID: 16084068. (n.d.).
Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014 Jan 1;75(1):9-17. doi: 10.1016/j.biopsych.2013.05.004. Epub 2013 Jun 18. PMID: 23786983; PMCID: PMC3858396. (n.d.).
Cassidy F, Ahearn E, Murry E, Forest K, Carroll BJ. Diagnostic depressive symptoms of the mixed bipolar episode. Psychol Med. 2000 Mar;30(2):403-11. doi: 10.1017/s0033291799001312. PMID: 10824660. (n.d.).
Cassidy F, Forest K, Murry E, Carroll BJ. A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry. 1998 Jan;55(1):27-32. doi: 10.1001/archpsyc.55.1.27. PMID: 9435757. (n.d.).
Chengappa KN, Levine J, Ulrich R, Parepally H, Brar JS, Atzert R, Brienzo R, Gopalani A. Impact of risperidone on seclusion and restraint at a state psychiatric hospital. Can J Psychiatry. 2000 Nov;45(9):827-32. doi: 10.1177/070674370004500907. PMID: 11143833. (n.d.).
Citrome L, Volavka J. Pharmacological management of acute and persistent aggression in forensic psychiatry settings. CNS Drugs. 2011 Dec 1;25(12):1009-21. doi: 10.2165/11596930-000000000-00000. PMID: 22133324. (n.d.).
Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009 Jan;9(1):55-71. doi: 10.1586/14737175.9.1.55. PMID: 19102669. (n.d.).
Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007 Dec;68(12):1876-85. doi: 10.4088/jcp.v68n1207. PMID: 18162018. (n.d.).
Conley RR, Kelly DL, Richardson CM, Tamminga CA, Carpenter WT Jr. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: a double-blind crossover study. J Clin Psychopharmacol. 2003 Dec;23(6):668-71. doi: 10.1097/01.jcp.0000096246.29231.73. PMID: 14624201. (n.d.).
Contreras-Rodríguez O, Pujol J, Batalla I, Harrison BJ, Soriano-Mas C, Deus J, López-Solà M, Macià D, Pera V, Hernández-Ribas R, Pifarré J, Menchón JM, Cardoner N. Functional Connectivity Bias in the Prefrontal Cortex of Psychopaths. Biol Psychiatry. 2015 Nov 1;78(9):647-55. doi: (n.d.).
Correll CU, Yu X, Xiang Y, Kane JM, Masand P. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017 May;29(2):92-107. PMID: 28463343. (n.d.).
Corrigan PW, Watson AC. Findings from the National Comorbidity Survey on the frequency of violent behavior in individuals with psychiatric disorders. Psychiatry Res. 2005 Sep 15;136(2-3):153-62. doi: 10.1016/j.psychres.2005.06.005. PMID: 16125786. (n.d.).
Cummings MA, Proctor GJ, Arias AW. Dopamine antagonist antipsychotics in diverted forensic populations. CNS Spectr. 2020 Apr;25(2):128-135. doi: 10.1017/S1092852919000841. Epub 2019 May 7. PMID: 31060635. (n.d.).
Cummings, M. (2015). The neurobiology of psychopathy: Recent developments and new directions in research and treatment. CNS Spectrums, 20(3), 200-206. doi:10.1017/S1092852914000741. (n.d.).
Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995 Aug;15(4):243-9. doi: 10.1097/00004714-199508000-00002. PMID: 7593706. (n.d.).
Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986 Jan-Feb;11(1):36-61. doi: 10.2165/00003088-198611010-00003. PMID: 2868820. (n.d.).
Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014 Jan 1;87(1):93-120. doi: 10.1016/j.bcp.2013.09.007. Epub 2013 Sep 17. PMID: 24055064. (n.d.).
Fazel S, Lichtenstein P, Frisell T, Grann M, Goodwin G, Långström N. Bipolar disorder and violent crime: time at risk reanalysis. Arch Gen Psychiatry. 2010 Dec;67(12):1325-6. doi: 10.1001/archgenpsychiatry.2010.171. Erratum in: Arch Gen Psychiatry. 2011 Feb;68(2):123. PMID: 21135334. (n.d.).
Fazel, S., Gulati, G., Linsell, L., Geddes, J. R., & Grann, M. (2009). Schizophrenia and violence: Systematic review and meta-analysis. PLoS Medicine, 6(8). https://doi.org/10.1371/journal.pmed.1000120
Fazel, S., Zetterqvist, J., Larsson, H., Långström, N., & Lichtenstein, P. (2014). Antipsychotics, mood stabilisers, and risk of violent crime. The Lancet, 384(9949), 1206–1214. https://doi.org/10.1016/s0140-6736(14)60379-2
Frogley C, Taylor D, Dickens G, Picchioni M. A systematic review of the evidence of clozapine's anti-aggressive effects. Int J Neuropsychopharmacol. 2012 Oct;15(9):1351-71. doi: 10.1017/S146114571100201X. Epub 2012 Feb 20. PMID: 22339930. (n.d.).
Goedhard LE, Stolker JJ, Heerdink ER, Nijman HL, Olivier B, Egberts TC. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: A systematic review. J Clin Psychiatry. 2006 Jul;67(7):1013-24. doi: 10.4088/jcp.v67n0702. PMID: 16889443. (n.d.).
Grace AA. The depolarization block hypothesis of neuroleptic action: implications for the etiology and treatment of schizophrenia. J Neural Transm Suppl. 1992;36:91-131. doi: 10.1007/978-3-7091-9211-5_6. PMID: 1356143. (n.d.).
Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43-62. Published 2014 Jun 23. doi:10.2147/PROM.S42735. (n.d.).
Haidary HA, Padhy RK. Clozapine. [Updated 2021 Dec 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535399/. (n.d.).
Haller J, Makara GB, Kruk MR. Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci Biobehav Rev. 1998;22(1):85-97. doi: 10.1016/s0149-7634(97)00023-7. PMID: 9491941. (n.d.).
Hankin CS, Bronstone A, Koran LM. Agitation in the inpatient psychiatric setting: a review of clinical presentation, burden, and treatment. J Psychiatr Pract. 2011 May;17(3):170-85. doi: 10.1097/01.pra.0000398410.21374.7d. PMID: 21586995. (n.d.).
Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annu Rev Clin Psychol. 2008;4:217-46. doi: 10.1146/annurev.clinpsy.3.022806.091452. PMID: 18370617. (n.d.).
Haslemo T, Olsen K, Lunde H, Molden E. Valproic Acid significantly lowers serum concentrations of olanzapine-an interaction effect comparable with smoking. Ther Drug Monit. 2012 Oct;34(5):512-7. doi: 10.1097/FTD.0b013e3182693d2a. PMID: 22972535. (n.d.).
Hoptman MJ. Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectr. 2015 Jun;20(3):280-6. doi: 10.1017/S1092852915000206. Epub 2015 Apr 22. PMID: 25900066; PMCID: PMC4441843. (n.d.).
Iozzino, L., Ferrari, C., Large, M., Nielssen, O., & de Girolamo, G. (2015). Prevalence and risk factors of violence by psychiatric acute inpatients: A systematic review and meta-analysis. PLOS ONE, 10(6). https://doi.org/10.1371/journal.pone.0128536
Jones RM, Arlidge J, Gillham R, Reagu S, van den Bree M, Taylor PJ. Efficacy of mood stabilisers in the treatment of impulsive or repetitive aggression: systematic review and meta-analysis. Br J Psychiatry. 2011 Feb;198(2):93-8. doi: 10.1192/bjp.bp.110.083030. PMID: 21282779. (n.d.).
Joshi A, Krishnamurthy VB, Purichia H, Hollar-Wilt L, Bixler E, Rapp M. "What's in a name?" Delirium by any other name would be as deadly. A review of the nature of delirium consultations. J Psychiatr Pract. 2012 Nov;18(6):413-8. doi: 10.1097/01.pra.0000422739.49377.17. PMID: 23160246. (n.d.).
Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006 Jun;63(6):622-9. doi: 10.1001/archpsyc.63.6.622. PMID: 16754835. (n.d.).
Lieberman JA, Safferman AZ, Pollack S, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. The American Journal of Psychiatry. 1994 Dec;151(12):1744-1752. DOI: 10.1176/ajp.151.12.1744. PMID: 7977880. (n.d.).
Longo, L. P., & Salzman, C. (1995). Valproic acid effects on serum concentrations of clozapine and norclozapine. American Journal of Psychiatry, 152(4). https://doi.org/10.1176/ajp.152.4.650a
Látalová K. Bipolar disorder and aggression. Int J Clin Pract. 2009 Jun;63(6):889-99. doi: 10.1111/j.1742-1241.2009.02001.x. PMID: 19490199. (n.d.).
Maden, T. (2004). Violence, mental disorder and public protection. Psychiatry, 3(11), 1–4. https://doi.org/10.1383/psyt.3.11.1.53590
Meyer J. Drug-drug interactions with antipsychotics. CNS Spectr. 2007 Dec;12(12 Suppl 21):6-9. doi: 10.1017/s1092852900015947. PMID: 18389925. (n.d.).
Meyer, J. (2014). A rational approach to employing high plasma levels of antipsychotics for violence associated with schizophrenia: Case vignettes. CNS Spectrums, 19(5), 432-438. doi:10.1017/S1092852914000236. (n.d.).
Meyer, J. M., Cummings, M. A., Proctor, G., & Stahl, S. M. (2016). Psychopharmacology of persistent violence and aggression. Psychiatric Clinics of North America, 39(4), 541–556. https://doi.org/10.1016/j.psc.2016.07.012
Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005 Jan;10(1):79-104. doi: 10.1038/sj.mp.4001556. PMID: 15289815. (n.d.).
Mohr P, Knytl P, Voráčková V, Bravermanová A, Melicher T. Long-acting injectable antipsychotics for prevention and management of violent behaviour in psychotic patients. Int J Clin Pract. 2017 Sep;71(9). doi: 10.1111/ijcp.12997. Epub 2017 Sep 4. PMID: 28869705. (n.d.).
Mohr, P., & Volavka, J. (2012). Adherence and long-acting injectable antipsychotics in schizophrenia: An update. Dusunen Adam: The Journal of Psychiatry and Neurological Sciences. https://doi.org/10.5350/dajpn20122504001
Newman, W. J. (2012). Psychopharmacologic management of Aggression. Psychiatric Clinics of North America, 35(4), 957–972. https://doi.org/10.1016/j.psc.2012.08.009
Pavlov KA, Chistiakov DA, Chekhonin VP. Genetic determinants of aggression and impulsivity in humans. J Appl Genet. 2012 Feb;53(1):61-82. doi: 10.1007/s13353-011-0069-6. Epub 2011 Oct 13. PMID: 21994088. (n.d.).
Pulay AJ, Dawson DA, Hasin DS, Goldstein RB, Ruan WJ, Pickering RP, Huang B, Chou SP, Grant BF. Violent behavior and DSM-IV psychiatric disorders: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2008 Jan;69(1):12-22. doi: 10.4088/jcp.v69n0103. PMID: 18312033; PMCID: PMC2922980. (n.d.).
Quanbeck, C. D., McDermott, B. E., Lam, J., Eisenstark, H., Sokolov, G., & Scott, C. L. (2007). Categorization of aggressive acts committed by chronically assaultive state hospital patients. Psychiatric Services, 58(4), 521–528. https://doi.org/10.1176/ps.2007.58.4.521
Sanne Wulff, Lars Hageman Pinborg, Claus Svarer, Lars Thorbjørn Jensen, Mette Ødegaard Nielsen, Peter Allerup, Nikolaj Bak, Hans Rasmussen, Erik Frandsen, Egill Rostrup, Birte Yding Glenthøj, Striatal D2/3 Binding Potential Values in Drug-Naïve First-Episode Schizophrenia Patients Correlate With Treatment Outcome, Schizophrenia Bulletin, Volume 41, Issue 5, September 2015, Pages 1143–1152, https://doi.org/10.1093/schbul/sbu220. (n.d.).
Stahl SM. Drugs for psychosis and mood: unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 2017 Oct;22(5):375-384. doi: 10.1017/S1092852917000608. PMID: 28965530. (n.d.).
Stahl, S., Morrissette, D., Cummings, M., Azizian, A., Bader, S., Broderick, C., . . . Warburton, K. (2014). California State Hospital Violence Assessment and Treatment (Cal-VAT) guidelines. CNS Spectrums, 19(5), 449-465. doi:10.1017/S1092852914000376. (n.d.).
Swanson JW, Swartz MS, Elbogen EB, Van Dorn RA. Reducing violence risk in persons with schizophrenia: olanzapine versus risperidone. J Clin Psychiatry. 2004 Dec;65(12):1666-73. doi: 10.4088/jcp.v65n1212. PMID: 15641872. (n.d.).
Swanson JW, Swartz MS, Van Dorn RA, Volavka J, Monahan J, Stroup TS, McEvoy JP, Wagner HR, Elbogen EB, Lieberman JA; CATIE investigators. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008 Jul;193(1):37-43. doi: (n.d.).
Swanson, J. W. (1994). Mental disorder, substance abuse, and community violence: An epidemiological approach. In J. Monahan & H. J. Steadman (Eds.), Violence and mental disorder: Developments in risk assessment (pp. 101–136). The University of Chicago Press. (n.d.).
Thomas K, Saadabadi A. Olanzapine. [Updated 2021 Aug 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532903/?report=classic. (n.d.).
Topiwala A, Fazel S. The pharmacological management of violence in schizophrenia: a structured review. Expert Rev Neurother. 2011 Jan;11(1):53-63. doi: 10.1586/ern.10.180. PMID: 21158555. (n.d.).
Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Predicting dopamine D₂ receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011 Jun;31(3):318-25. doi: 10.1097/JCP.0b013e318218d339. PMID: 21508857. (n.d.).
Urban AE, Cubała WJ. Therapeutic drug monitoring of atypical antipsychotics. Psychiatr Pol. 2017 Dec 30;51(6):1059-1077. English, Polish. doi: 10.12740/PP/65307. Epub 2017 Dec 30. PMID: 29432503. (n.d.).
Van Dorn, R., Volavka, J., & Johnson, N. (2011). Mental disorder and violence: Is there a relationship beyond substance use? Social Psychiatry and Psychiatric Epidemiology, 47(3), 487–503. https://doi.org/10.1007/s00127-011-0356-x
Veerman SR, Schulte PF, de Haan L. The glutamate hypothesis: a pathogenic pathway from which pharmacological interventions have emerged. Pharmacopsychiatry. 2014 Jul;47(4-5):121-30. doi: 10.1055/s-0034-1383657. Epub 2014 Jul 7. PMID: 25002292. (n.d.).
Veerman SRT, Schulte PFJ, de Haan L. Treatment for Negative Symptoms in Schizophrenia: A Comprehensive Review. Drugs. 2017 Sep;77(13):1423-1459. doi: 10.1007/s40265-017-0789-y. PMID: 28776162. (n.d.).
Volavka J, Citrome L. Pathways to aggression in schizophrenia affect results of treatment. Schizophr Bull. 2011 Sep;37(5):921-9. doi: 10.1093/schbul/sbr041. Epub 2011 May 11. PMID: 21562140; PMCID: PMC3160235. (n.d.).
Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002 Feb;159(2):255-62. doi: 10.1176/appi.ajp.159.2.255. Erratum in: Am J Psychiatry 2002 Dec;159(12):2132. PMID: 11823268. (n.d.).
Volavka J. Violence in schizophrenia and bipolar disorder. Psychiatr Danub. 2013 Mar;25(1):24-33. PMID: 23470603. (n.d.).