Episode 179: Exercise & Mental Health 2023 Update
By listening to this episode, you can earn 2.0 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Rachel Lockard MD, MPH, Desiree Turner, Manal Piracha, David Puder, MD
In a previous episode of the podcast, we discussed exercise for the brain, reviewing the pathophysiology between exercise and dementia, the pathophysiological mechanisms associated between low skeletal muscle mass and cognitive function, exercise as a treatment, and cardiorespiratory fitness and its relationship to all-cause mortality. In today’s episode, we look at the extensive research available on these subjects.
Key Findings From Previous Episode
Overall Health Benefits of Strength Training:
Exercise may lead to improved memory function due to reversing hippocampal volume loss, leading to improvements in spatial memory (Erikson, 2011).
Low skeletal muscle has also been associated with cognitive impairment and dementia in older adults, which may be due to the effects of exercise on systemic inflammation, insulin metabolism, protein metabolism, and mitochondrial function (Oudbier, 2022).
Systemic inflammation occurs with aging, as there is an increase in pro-inflammatory cytokines and a decrease in myokines, which decreases neuroprotective factors such as BDNF.
This Week’s Episode
Neurobiological Effects of Exercise
This review from De Sousa et al. (2021) describes how physical exercise enhances insulin-like growth factor 1 (IGF-I) and activates PGC-1α/FNDC5/Irisin pathway. Physical exercise also increases expression of brain-derived neurotrophic factor (BDNF) and its receptor, TrkB, in the hippocampus and prefrontal cortex leading to upstream of ERK and inhibiting depressive-like behavior. They show that as IGF-1 increased, antidepressant effects and increases in BDNF were seen in mice. BDNF neurotrophin is an immediate upstream regulator of extracellular signal-regulated kinase (ERK).
Figure from De Sousa et al. (2021).
Marinus et al. (2019) conducted a meta analysis of 17 studies that revealed strength training and combined aerobic/strength training increases peripheral blood BDNF concentrations in older adults (Z=2.94, P=0.003, Z=3.03, P=0.002, respectively), while low-to-moderate aerobic exercise alone does not (Z=0.82, P=0.41) (Marinus et al., 2019).
Wu et al. (2022) conducted a meta-analysis of fMRI studies looking at the effects of exercise on inhibitory control (14 studies, n=397). Impaired inhibitory control is seen in ADHD, bipolar disorder, schizophrenia, and substance use disorders. They used activation likelihood estimates to find clusters of exercise-induced neuronal changes in the superior frontal and precentral gyri in frontal lobe, precuneus region in the parietal lobe (essential for response to conflict and attention selection), the temporal gyrus and caudate in the temporal lobe, and the posterior cingulate gyrus and parahippocampal gyrus in the limbic system.
This study highlights connectivity in the frontoparietal network with activation in the frontal lobe associated with motor and cognitive control and the precuneus region of the parietal being essential for attention selection. They also note the importance of regions in the temporal lobe being active during inhibitory control tasks. They also propose that the posterior cingulate gyrus and parahippocampal gyrus of the limbic system structures underlie the mechanism of exercise improvement of cognitive control function through spatial information processing and object recognition, as well as being one of the locations of changes in BDNF levels (Wu et al., 2022).
Baseline Strength, Physical Activity, and Depression
Mahmoudi et al. (2022) conducted a systematic review and meta-analysis studying aerobic and resistance training on depressive symptoms, quality of life, and muscle strength in older adults (>60 years). This included 18 studies of older adults without regular exercise at baseline that had a pre-post exercise study design with a non-exercise control (n=1,354, mean age 65-85). They found that exercise training significantly declined depressive symptoms and led to a significant reduction in bodily pain and body mass. This was mediated by significant increases in mental health, physical functioning, and general health subscales of quality of life as well as upper and lower limb strength.
Marques et al., 2020 conducted a cross-sectional and prospective study of late middle-aged to older adults (n=32,392) and moderate or vigorous physical activity (PA) and depression symptoms. The study included 14 European countries across a 4-year follow-up. Moderate and vigorous PA at least once a week is negatively related to depression. This remained significant in the fully-adjusted model including self-rated health, sociodemographic characteristics, and the presence of chronic diseases. Despite initial PA, being active in the present significantly decreases the odds of having current depressive symptoms.
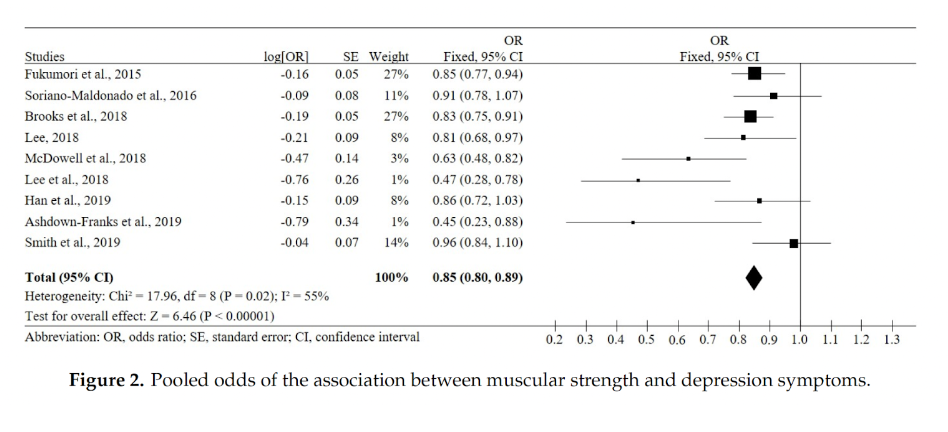
When looking at studies including adults of all ages, a systematic review and meta-analysis including 21 studies (n= 87,508 adults aged ≥18 years, from 26 countries) comparing muscular strength and depression symptoms showed that increased strength was associated with reduced depression symptoms with a pooled OR of 0.85 (Marques et al., 2020).
Figure from Marques et al. (2020).
Overall physical strength can be estimated using handgrip strength. Multiple studies have shown an association with weaker handgrip strength and the incidence of depression and anxiety.
In a cohort study involving 6,392 adult Chinese participants, the incidence of depressive symptoms was higher in populations with lower handgrip strength. In this cohort study, the incidence of depression was 11.9%, 15.5%, and 22.1% in the strong, moderate, and weak handgrip strength group, respectively. This is a consistent finding across countries. The same authors performed a meta-analysis of 6 prospective studies involving 26,473 participants and found a significantly decreased risk of depression symptoms among participants with strong handgrip strength (RR=0.74) (Huang et al., 2019).
A study from Cabanas-Sanchez et al. included 162,167 participants with a mean age of 56 (range 38-70). 3.4% developed depression and 4.1% developed anxiety over 10.0 years. Depression and anxiety outcomes were extracted from primary care and hospital admission records. The stronger the handgrip, the less likely one was to develop depression or anxiety during the follow-up (Cabanas‐Sánchez et al., 2022). The Longitudinal Ageing Study in India (LASI) was a cross-sectional study with older adults aged 60 years and above (n=27,707) and confirmed findings that weaker handgrip strength was associated with depression and cognitive impairment (Muhammad & Maurya, 2022).
Figures from Cabanas‐Sánchez et al., (2022).
A review and network meta-analysis from Yu et al. (2022) of multiple forms of exercise on specific mental health conditions included 117 RCTs of patients with a single diagnosis of depression, anxiety, PTSD, or schizophrenia (n=6,456 participants). Network meta-analysis allows for comparing relative effectiveness of multiple interventions simultaneously and ranking treatments. Multimodal exercise (aerobic, resistance, mind-body) ranked best for treating depression (SMD = 2.7), but aerobic exercise and mind-body exercises both had large effect sizes (>0.78). Resistance exercise seemed to be more beneficial for those with anxiety-related disorders. Positive and negative schizophrenic symptoms were separated in analysis, with resistance exercise having the highest probability of treating positive symptoms and multimodal exercise being ranked highest for negative symptoms (Yu et al., 2022).
Increasing Strength for Depression
A recent systematic review and meta-analysis with meta-regression was conducted by Heissel et al. (2023) of exercise on major depressive disorder (MDD) and depressive symptoms included 41 studies, n=2,264 adult participants post-intervention. They excluded studies with mind-body exercises and interventions that included other standard treatments (medication, psychotherapy) unless treatment was < 3 months prior. Results showed moderate-to-large effects of exercise on depressive symptoms even when limiting the analysis to low risk of bias studies or only MDD. The main analysis of pooled data from 41 studies showed a large effect size (SMD = −0.946), which increased when excluding studies <6 weeks of intervention (SMD = −0.959). Both aerobic (SMD = −1.156) and resistance training (−1.042) independently showed large effects, whereas mixed aerobic and resistance training showed small effects (−0.455). The calculated NNT was 2.0 for the main-analysis, 2.8 for the low risk of bias studies, 1.9 for MDD only, and 1.6 in supervision by other professionals/students. They also showed that non-inferiority trials indicated that exercise is non inferior to current first-line treatments.
A recent umbrella review of systematic reviews of physical activity interventions and mental health conditions (including depression, anxiety, psychological distress) by Singh et al. (2023) included 97 reviews, 1,079 RCTs, and 128,119 participants. PA across all participants groups and interventions had medium effects on depression (ES=−0.43), anxiety (ES=−0.42), and psychological distress (ES=−0.60). The largest benefits were seen in people with depression, HIV and kidney disease, in pregnant and postpartum women, and in healthy individuals. Similarly, higher intensity physical activity was associated with greater improvements in symptoms. Larger effect sizes were observed in clinical populations with above-average symptoms of depression and anxiety (Singh et al., 2023).
Barahona-Fuentes et al. (2021) conducted a systematic review and meta-analysis of adolescents and strength training interventions on depression, anxiety, or stress. Nine studies were included in the systematic review (described strength training interventions in reps/intensity) and seven in the meta-analysis (n=448). They found a large and significant effect of strength training on anxiety (SMD = −1.75) and depression (SMD = −1.61). If you remove studies of aerobic + strength training (SMD = –1.33), conventional strength training only has a higher effect (SMD = –1.92) on depression.
Fabiano et al. (2023) conducted a systematic review and meta-analysis of effects of exercise interventions on suicidal ideation and behaviors (17 studies, n=1702 participants, mean follow-up 10 weeks). Post-intervention effect on suicidal ideation was not different between exercise and control groups (SMD = -1.09), but there were improvements seen in number of suicide attempts (OR = 0.23).
Moraes et al. (2020) conducted a RCT with older persons clinically diagnosed with MDD (n = 27) and treated with antidepressants. Groups were randomized into 3 groups for 12 weeks: (1) Aerobic training (AT): 5 min warm up, 20 minutes of 70% HR max or 60% of VO2 max., 5 min warm down; (2) Strength training (ST): 3 sets 8-12 reps of chest press, low row, leg extension, leg curl; and (3) Low-intensity exercise in a control group (CG). In the Hamilton Depression Scale (HAM-D), both strength and aerobic beat control. No difference was found between the aerobic group and strength groups. A limitation of the study is that they excluded more significant depression (HAM-D greater than 18 points).
Reviews of the literature for Episode 96 released in 2020 show that increasing strength has a large impact on depression. A large meta-analysis of 33 randomized controlled trials involving 1,877 patients showed that resistance exercise training significantly reduced their depressive symptoms, with a mean effect size of 0.66. The number needed to treat for 1 patient to enter remission was 4 (Gordon, 2018). A meta-analysis of 27 randomized controlled trials involving 1,452 clinically depressed adults revealed that strength training decreased depressive symptoms more than endurance training did, with a standard mean difference of -0.96 for strength training and -0.52 for endurance training. Endurance training lasting longer than 10 minutes increased the effect size to -0.62 (Nebiker, 2018).
A randomized controlled crossover trial of 68 youth (15-25 years old) with major depressive disorder (MDD) were assigned to multimodal exercise of resistance training and high-intensity interval training for 12 weeks. No significant association between strength/aerobic exercise attendance and change in depression severity was observed, but there was a dose-dependent inverse relationship between bench press repetitions and depression severity, with an effect size of -0.51 (Nasstasia, 2019).
High Intensity Interval Training (HIIT) Interventions
Reviews for Episode 96 included a large population study from Bennie et al. (2019) of 17,839 adults that found the combination of aerobic and muscle-strengthening exercise was associated with the lowest likelihood of reporting depressive symptoms, followed by aerobic exercise only and muscle strengthening only. Prevalence ratios were 0.26-0.54, 0.36-0.62, and 0.49-0.84, respectively (Bennie, 2019). A cross sectional study from Oftedal et al. (2019) of 5,180 Australian women found that a combination of resistance training and aerobic exercise yielded lower probabilities for depression (RRR=0.61 [0.43-0.86]) than aerobic exercise alone (Oftedal, 2019).
For more recent studies looking at HIIT interventions alone, anxiety symptoms were most commonly studied with improvements seen in clinical anxiety symptomatology. In a limited trial (n=33 participants) by Plag et al. (2020), HIIT was compared to low intensity training (LIT) for treatment of generalized anxiety disorder (GAD). Effects of HIIT were generally about twice as high as for LIT in reducing symptoms of GAD.
A systematic review and meta-analysis assessing exercise and clinical anxiety looked at fifteen studies (n= 675 patients). Results indicated that higher intensity exercise showed more effectiveness than lower intensity for reduction in symptoms of anxiety (Aylett et al., 2018). HIIT for PTSD has been evaluated in multiple small studies with individuals with trauma exposure and subclinical reporting of PTS symptoms. These are extremely limited studies that appear to be cautiously testing the utilization of HIIT with PTSD.
To better understand the effects of various forms of physical activity on strength, Schoenfeld et al. (2017) conducted a meta-analysis (N=21 studies) comparing changes in strength and hypertrophy between low- vs. high-load resistance training protocols. Outcomes for strength found that heavy loading had a greater advantage for gains in 1 repetition maximum strength, compared with low-load training. However, both heavy and light loads showed large effects for 1 repetition maximum increases of about 35.4% and 28.0%, respectively. Heavy loads are essential to reaching maximal gains in isotonic strength, but lighter loads also contribute to substantial increases in this outcome. High and low loads produced similar gains in isometric strength, mean percentage changes were 22.6% and 20.5%. In terms of muscle size, high and low load conditions found similar hypertrophic changes. The mean percentage gains were 8.3% vs 7.0%. Therefore, both heavy and low loads can help promote muscle growth, given that their training is carried out with a high amount of effort (Schoenfeld et al., 2017).
Exercise and Resilience, Stress-Reactivity, & Fear Extinction
Resilience allows one to maintain mental health despite exposure to psychological or physical adversity. Resilience research focuses on protective mechanisms that shield people against the development of stress-related disorders. Fear extinction is the decline of a fear response after repeated exposure to a fear-eliciting conditioned stimulus and is a target for treatment of trauma-related disorders. Impaired resilience or fear extinction contributes to the persistence of many stress- or trauma-related disorders such as anxiety and PTSD.
A longitudinal-prospective study (n=431 adults) by Neumann et al. (2022) compared physical fitness and resilience. They excluded schizophrenia or bipolar disorder, organic mental disorders, substance dependence, and severe axis-I disorders. Fitness was measured with cardiorespiratory fitness (CRF measured as VO2Max), composite strength (handgrip, standing long jump), and sum of metabolic equivalent (MET)-minutes per week (high >3000). Exercises were described through time walking, moderate activity (e.g., carrying light loads, bicycling, or easy swimming), vigorous activity (e.g., lifting, aerobics, fast running). Resilience was indexed by stressor reactivity in response to critical life events and daily hassles, monitored quarterly for 9 months. They found that both muscular and self-perceived fitness were positively associated with stress resilience. Self-reported physical activity and CRF did not independently predict stress resilience. To better understand this, they examined the impact of self-efficacy and found that the muscular fitness and stress resilience relationship was mediated by general self-efficacy. This was proposed to the relation of one’s own perception of fitness and closely relates to the concept of self-efficacy and capability of acting (Neumann et al., 2022).
Szuhaney et al. (2023) recently conducted a cohort study of older adults (50+) who experienced at least one stressor: child/spousal bereavement, myocardial infarction (MI), divorce, job loss, or disability (n=1405) and depression trajectories (resilient, improving, emerging, chronic). Using linear modeling, they found that baseline exercise predicted resilience following a stressor. The resilient group had higher exercise levels pre-to post-stressor. The improving depression group had consistent moderate exercise levels. Increasing and stable-high depression groups reduced exercise following a stressor (Szuhany et al., 2023).
Tanner et al. (2019) provide a narrative review of exercise modulation of fear extinction. The animal data indicates a lack of a beneficial effect of chronic exercise on fear extinction under typical experimental conditions and postulates that this is due to memory consolidation, including consolidation of contextual fear conditioning. Inversely, acute exercise can enhance fear extinction even in previously sedentary rats, and exercise occurring during or immediately following fear extinction is unique in that it not only enhances fear extinction memory, it also reduces fear relapse. Mechanistically, they suggest that nigrostriatal dopamine could contribute to exercise-augmentation of fear extinction. This has been shown through central norepinephrine and dopamine circuits being recruited during exercise, as well as having been reported to enhance fear extinction and increase BDNF (Tanner et al., 2019).
Moya et al. (2021) utilized a rodent model to study mTOR in fear-extinction learning, memory consolidation, and neuronal plasticity following acute exercise. They performed an intracerebral-ventricular injection of rapamycin prior to fear extinction training and acute exercise (wheel running). Literature has shown inhibition of the mTOR pathway with rapamycin decreases p70s6 kinase expression in the amygdala and impairs consolidation of auditory fear memories. They showed that rapamycin injections reduced immunoreactivity of phosphorylated S6 in brain regions involved in fear extinction and eliminated the enhancement of fear extinction memory produced by acute exercise, without reducing voluntary exercise behavior or altering fear extinction in sedentary rats (Moya et al., 2021).
PTSD and Exercise
A small pilot, RCT from Powers et al. (2015) (n=9, 8 females, avg age = 34) included participants with PTSD and randomized them to prolonged exposure (PE) therapy alone or PE + exercise (30-minute bout of moderate-intensity treadmill). They hypothesized that fear extinction modulation could improve outcomes in exposure therapy and that interventions modulating BDNF could play a positive role. Through a mechanistic review, they describe research showing synaptic plasticity in brain regions that are involved in the consolidation of fear extinction and that in humans, reduced BDNF release is associated with impaired functioning of extinction circuitry. Their small study showed that chronic exercise augmented exposure therapy with improvements in PTSD symptoms and elevated BDNF (Powers et al., 2015).
Jadhakhan et al. (2022) conducted a systematic review of exercise on adults with PTSD, including 13 studies from four countries (n=531). Individual forms of exercise/physical activity examined showed some effect on reducing PTSD symptoms, but combined exercises (resistance training, aerobic, strength and yoga) administered over a 12-week period, three times a week for 30–60 min showed greater effects on PTSD symptoms (Jadhakhan et al., 2022).
Reis et al. (2022) conducted a systematic review including 6 single-arm studies (n=101 participants, rated with serious risk of bias) and 3 RCTs (n=217 participants, some concern of elevated bias) where most single-arm studies used yoga-based interventions, whereas RCTs showed more variety and included yoga, aerobic activity, and resistance exercises. They explored exercise, as evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans but are accompanied by high rates of dropout (eg, 40–60%). The pooled SMD of −0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of −0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions. Given results, there is still a need for additional high-quality randomized trials to confirm the benefits of exercise for PTSD symptom reduction in veterans.
A narrative review of aerobic exercise interventions (+/- resistance training) by Hegberg et al. (2019) included 19 relevant studies (9 observational, 10 intervention). They found that both observational and intervention studies provide support for the notion that aerobic exercise, either alone or in combination with standard treatments, exerts positive mental health benefits among individuals with PTSD.
In one prospective study of 38,883 randomly selected U.S. veterans (77% male), 96% of which did not have PTSD at baseline, participants who reported engaging in vigorous exercise (≥ 20 min, two or more times per week) had significantly decreased odds of developing new PTSD symptoms (OR= 50.58) or having persistent symptoms (OR 50.59) at 3–5 year follow-up (LeardMann et al., 2011).
In another study, inpatients with PTSD (N = 81; mean age: 48; 84% male; BMI = 30.1 obese) were assigned to either a 12-week exercise program that included three, 30-min sessions per week of progressive resistance training, walking (daily step count goal = 10,000 steps), and “care as usual” to “care as usual” alone. Individuals in the exercise group had significantly greater symptom reduction on a self-report measure of PTSD (mean difference = −5.4) (Rosenbaum et al., 2015).
They suggest potential mechanisms by which aerobic exercise could exert a positive impact in PTSD include exposure and desensitization to internal arousal cues, enhanced cognitive function, exercise-induced neuroplasticity, normalization of hypothalamic pituitary axis (HPA) function, and reductions in inflammatory markers. Specifically, repeated exposure to physiological cues, such as rapid heartbeat, in the context of exercise may increase tolerance of and facilitate desensitization to the physiological sensations (Hegberg et al., 2019).
Physical Activity And Anxiety Disorders
Zika & Becker (2021) conducted a systematic review with multiple meta-analyses of social anxiety disorder (SAD) and any exercise intervention (excluding mind-body or relaxation-focused) for comparisons between interventions (i.e. group vs. individual, endurance vs. resistance training, with or without the presence of a therapist/trainer) with either passive or active control (ex. CBT).
Meta-analysis of studies that included a control group consisted of 4 studies (n=749 participants, mean age 14, range 9-32) had a pooled effect that was not significant, but SAD symptoms were lower in the PA group, subgroup analysis of pooled effect for fear of negative evaluation (FNE) was medium in size (d = −0.48). Twelve studies were included in meta-analysis without a control group (n=29,333, average age 21, range 8-38) with significant medium-sized effect (d = −0.22). 13 studies were included in meta-analysis of cross-sectional studies; the pooled effect size was small, but significantly different from zero. Further subgroup analysis showed significantly lower SAD in longitudinal studies and that the effect of PA was stronger for adults than for children and adolescents. For cross-sectional studies, lower SA was found for people who were more physically active. They believe that lower effect sizes could be due to low levels of SAD symptomatology and FNE reported at baseline in studies, potentially due to fear of participating in long-term study (Zika & Becker, 2021).
Dong et al. (2022) conducted an cross-sectional study of college students (n=248) looking at executive function via Stroop test, the State-trait Anxiety Inventory (STAI), then self-reported physical activity (PA). They were interested in the mediating effects on trait anxiety. Anxiety is divided into both state and trait anxiety. (State anxiety is a transient response, whereas trait anxiety is relatively stable associated with anxiety disorders and depression, and poorer executive function.) Vigorous physical activity had a direct effect on low trait anxiety, while the effect of moderate physical activity and low physical activity was mediated exclusively through executive function. Physical activity level had a 72% direct effect on reducing trait anxiety and promoted working memory and inhibition function (Dong et al., 2022).
Effectiveness of CBT for anxiety disorders is well documented and psychotherapies for anxiety disorders yield large effect sizes with CBT ranging from 1.3–1.22 (Bandelow et al., 2015) but still experience high remission rates. To evaluate exercise as an adjunct therapy for CBT, they analyzed eight studies with a total of 431 adult subjects. Add-on PE seems to be feasible and more beneficial for clinical populations when administered regularly several times per week, across several weeks (Frederiksen et al., 2021).
Schizophrenia and Exercise
Bredin et al. (2021) conducted a review and meta-analysis of effects of aerobic, resistance, and combined aerobic and resistance exercises (excluded HIIT and mind-body) on schizophrenia symptoms (Positive and Negative Syndrome Scale). 22 studies (n=913) included in review and 12 studies (n=554) included in meta-analysis.
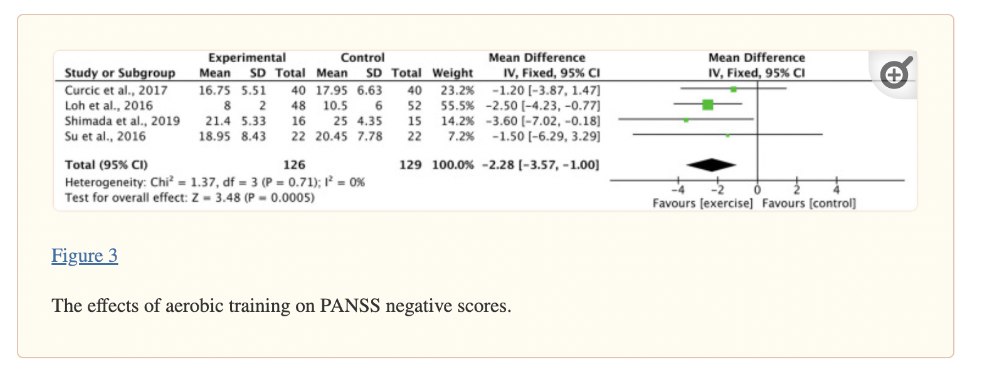
Aerobic training had a significant decrease in negative PANSS scores (ES −2.28) and total (ES −2.51), though recent meta-analysis of just aerobic exercises found benefits with positive PANSS scores, as well (Sabe, 2020). Resistance training alone did not lead to significant effects on PANSS (n=3 studies), although interventions were short (average of 12 weeks), the sample sizes were small (average of 12 participants in the RT groups), and the RT interventions themselves were limited. Grouping together the results from all exercise training modalities revealed significant effects on PANSS negative symptoms (ES −1.90) and Scale for the Assessment of Negative Symptoms (SANS) total (ES −14.90) (Bredin et al., 2021).
Exercise as a Treatment for Schizophrenia is a narrative review of exercise for schizophrenia from Girdler et al. (2019) conducted due to potential neurobiological mechanisms and prior research showing improvements of multiple forms of exercise on symptom severity. Dauwan et al. (2016) (large meta-analysis, n=1,109) showed that physical exercise (PE) had an overall significant effect on improving total symptom severity and both negative and positive symptoms with the largest effect size for negative symptoms. Another review from Firth et al. (2017) (10 studies, n=385 participants) showed that PE improved social cognition, working memory, and attention in patients with schizophrenia and that exercise programs supervised by physical activity professionals were significantly more effective (Girdler et al., 2019).
Mechanistically, patients with schizophrenia have also been found to have decreased serum levels of BDNF. Resistance and aerobic exercise induced increased serum levels of BDNF as well as improved neurocognitive functioning in patients with schizophrenia after 12 weeks of an exercise program (Kim, 2014). Structurally, Pajonk et al. (2010) showed that 3 months of aerobic exercise (moderate-intensity cycling) increased hippocampal volume by 12% compared to no improvement in the non-exercise group resulting in improvements in short-term memory (Girdler et al., 2019).
A systematic review of trials investigating strength training in schizophrenia spectrum disorders, a study aimed at specifically comparing strength training to other forms of exercise, resulted in a review of five studies. At that time only two studies examined the impact of isolated strength training in patients with schizophrenia (Keller-Varady et al., 2019).
RCT with machine-based strength training for large muscle groups (leg press, leg curl, vertical traction, chest press, arm extension, arm curl, abdominal crunch) reported a significantly improved muscle strength and psychopathology (PANSS), but no statistically significant effect on depressive symptoms, general quality of life, or BDNF serum levels (Silva, 2015). Strength training in patients with gait deficits showed that leg press did not improve psychopathology or quality of life measures, but improved impaired walking performance (Heggelund, 2012). Very limited sample sizes in both studies with Silva et al. n=12 for RE group and Heggelund et al. n=6 for ST group (Keller-Varady et al., 2019).
Physical exercise is known to be a key component in improving sleep quality among the general public. Poor sleep quality can have a significant impact among individuals with severe mental illness. Patients with schizophrenia may experience a worsening of their symptoms due to poor sleep quality and poor sleep quality effects include worsening cognition and function during waking hours, contributing to symptoms of metabolic syndrome and cardiovascular disease (Subotnik et al., 2023).
Participants in the study completed an average of 12.6 group exercise sessions and 12.9 individual at home exercise sessions. Their sleep was assessed at baseline and again at 6 months. The study found that the number of group exercise sessions during months 4-6 was associated with longer sleep duration, less dysfunction during the daytime due to lack of sleep, and non-significantly with less need for sleep medication. The group exercise found an improvement in total sleep quality (p= 0.008), however individual exercise was not significantly predictive of total sleep quality (p=0.19) (Subotnik et al., 2023).
Exercise For ADHD
Liang et al. (2021) conducted a systematic review (21 studies) and meta-analysis (15 studies, n=493) of RCTs studying exercise interventions and executive function in children and adolescents with ADHD. Exercise interventions improved overall executive function (SMD = 0.611) and had a moderate-to-large positive effect on inhibitory control (g = 0.761) and cognitive flexibility (g = 0.780). Moderate (g = 0.797) to vigorous PA (g = 1.426) and chronic exercise (g = 0.789) significantly moderated effect size (Liang et al., 2021).
The goal of this study by Kadri et al. (2019) was to investigate the effects of Taekwondo intervention of cognitive function in adolescents with ADHD. Taekwondo training specifically incorporates physical and mental components which have shown to increase brain activity and connectivity. The intervention was one and a half years long and the two cognitive instruments that were used to assess cognition were the Stripp and the Ruff 2 and 7 tests. The post test scores between the Taekwondo group and the control group showed statistically significant differences. The Stroop color block test had a large effect size of 1.26. The color word interference test had an effect size of 2.16. The interference test had an effect size of 1.63. Similar results were found in the Ruff 2 and 7 test (measures different components of attentional processes), with an effect size of 2.78 (Kadri et al., 2019).
Borderline Personality Disorder & Exercise
Mehren et al. (2020) described the potential benefit of physical activity for the treatment of borderline personality disorder. There is incredibly limited research into exercise and BPD but they use shared symptomatology between ADHD and BPD to show how exercise affects selected BPD-relevant symptoms. Both, ADHD and BPD are characterized by affective instability and impulsive behavior and impairments in executive functioning with benefits in those domains being seen in treatment of ADHD. As in ADHD, exercise-related release of catecholamines could be a potential mechanism of action in BPD, not only improving executive functioning and reducing impulsivity, but also influencing mood-related symptoms. Exercise has shown to affect the endogenous opioid system and to enhance mental stress sensitivity and therefore might positively impact those symptoms in BPD as well (Mehren et al., 2020).
St-Amour et al. (2022) conducted a small RCT looking at the effect of physical exercise on negative affect in patients with BPD. They randomly assigned 28 participants with BPD to a 20-minute single session of stationary bicycle or a control condition (emotionally neutral video) then watched a short video clip simulating negative mood induction (Silence of the Lambs). Following the negative mood induction, both conditions decreased the level of negative affect with a medium effect size, but there was no significant difference between them (St-Amour, 2022).
Exercise Interventions on Autism Spectrum Disorders (ASD)
Liang et al. (2022) conducted a systematic review (4 articles) and meta-analysis (7 articles, n=248) studying effects of exercise on executive function and cognition in children and adolescents with autism spectrum disorders. Chronic exercise interventions had a small to moderate positive effect on overall EFs in children and adolescents with ASD (g = 0.342). Specifically, chronic exercise interventions had a small to moderate positive effect on cognitive flexibility (g = 0.312) and inhibitory control (g = 0.492), but a non-significant effect size (g = 0.212) on working memory (Liang et al., 2022).
A systematic review and meta-analysis from Yang et al. (2022) on physical activity interventions and mental health and cognition for child and adolescents with intellectual disabilities included 15 studies, n=630 participants with mean age of 13 years. They found significant and large effects of physical activity on mental health in children and adolescents with IDs (g = 0.9), with medium effects on psychological health (g = 0.54) and large effects on cognitive function (g = 1.2). RCT design and intervention components (> 120 minutes per week, therapeutic, and aerobic exercise) demonstrated the strongest effects (Yang et al., 2022).
Addiction/Substance Use Disorder (SUD) and Exercise Interventions
Giménez-Meseguer et al. (2020) conducted a systematic review and meta-analysis (59 studies, n=3,792) examining the effects of physical exercise on mental disorders, quality of life, abstinence, and craving. They found a positive effect of exercise on mental disorders (SMD = 0.66) and quality of life (SMD = 0.69). Subgroup analysis revealed an effect of exercise in craving (SMD = 0.80), stress (SMD = 1.11), anxiety (SMD = 0.50) and depression (SMD = 0.63). When looking at types of exercise, aerobic and combinations of aerobic and strength were effective for the improvement of physical conditions in drug-dependent patients (Giménez-Meseguer, 2020).
Rates Of Injury In Strength Sports
Importantly, resistance training (including weightlifting and powerlifting) has not been shown to have increased rates of injury when compared to other sports. In a systematic review from Aasa et al. in 2017, the risk of injury in weightlifting was 2.4-3.3 injuries/1000 hours of training and 1.0-4.4 injuries/1000 hours of training in powerlifting, which is comparable to other non contact sports. The risk of injury in weightlifting and powerlifting is considerably less than contact sports such as football, which has 9.6 injuries/1000 hours of training (Aasa et al., 2017). This is in comparison to other non contact sports such as competitive soccer, which has a risk of injury of 53 injuries/1000 hours of training (Rahnama, 2002).
Bone Health
Resistance training is beneficial for any gender and any age group. Bi-weekly resistance training done over a year showed either maintained or increased bone mineral density in postmenopausal women. When resistance training is combined with weight-bearing impact aerobic exercise such as jogging, bone mineral density is maintained or increased in both older women and men (Hong, 2018).
Antipsychotic Treatment & Metabolic Syndrome
Pillinger et al. (2020) conducted a systematic review and meta-analysis of blinded RCTs comparing 18 antipsychotics and placebo in the acute setting of schizophrenia (100 studies; n= 25,952 patients). A random-effects network meta-analysis was conducted to investigate treatment-induced changes in body weight, BMI, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, and glucose concentrations. Antipsychotics vary markedly in their effects on body weight, BMI, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and glucose concentrations.
Change in weight (83 studies, n=18,750 participants using 18 antipsychotics and n=4,210 with placebo): Evidence of weight gain with brexpiprazole, risperidone and paliperidone, quetiapine, iloperidone, sertindole, olanzapine, zotepine, and clozapine; Haloperidol ranked the best and Clozapine ranked the worst.
Change in BMI (22 studies, n=4,196 participants using 9 antipsychotics, n=900 placebo): No change in BMI was observed with haloperidol or aripiprazole. BMI increased with lurasidone, risperidone, paliperidone, quetiapine, sertindole, clozapine, and olanzapine. Haloperidol ranked the best and Olazapine ranked the worst in terms of degree of associated BMI alteration.
Change in cholesterol (36 studies, n=11,762 participants using 14 antipsychotics and n=2,998 with placebo): Total cholesterol increased with quetiapine, olanzapine, and clozapine; Cariprazine ranked as the best and clozapine as the worst.
Change in LDL cholesterol (24 studies, n=7,439 participants using nine antipsychotics, n=2,419 with placebo): Observed decrease in LDL cholesterol with cariprazine and increases in LDL cholesterol with quetiapine and olanzapine; cariprazine ranked the best and olanzapine the worst.
Change in HDL cholesterol (22 studies, n=7,073 participants using ten antipsychotics, n=2,189 with placebo): HDL cholesterol increased with aripiprazole and brexpiprazole; aripiprazole and brexpiprazole ranked the best and amisulpride ranked the worst.
Change in triglycerides (34 studies, n=10,965 participants using 15 antipsychotics, n=3,021 with placebo): Compared with placebo, triglyceride concentrations increased with quetiapine, olanzapine, zotepine, and clozapine.
Change in fasting blood glucose (37 studies, n=10,681 participants using 16 antipsychotics, n=3,032 placebo): Glucose concentrations reduced with lurasidone, and increased with olanzapine, zotepine, and clozapine.
Overall Findings:
Clozapine and olanzapine are associated with the largest degree of metabolic dysregulation.
Some of the drugs were shown to perform better than placebo on some metabolic measures: lurasidone led to reductions in glucose, cariprazine to reductions in LDL cholesterol, aripiprazole and brexpiprazole to increases in HDL cholesterol.
Cardiovascular Health, Diabetes Prevention, & Weight Loss
It is acknowledged that multiple forms of exercise show benefits in cardiovascular health and weight loss. A meta-analysis comparing aerobic training alone and combined aerobic and resistance training in patients with coronary artery disease found combined training superior in decreasing body fat percentage, increasing fat-free mass, and increasing VO2max compared to aerobic training alone (Marzolini, 2020).
Diabetes prevalence has more than doubled since 1980, with 153 million cases increasing to more than 400 million in 2015, according to the World Health Organization. Type 2 diabetes mellitus is characterized by mitochondrial dysfunction and insulin resistance leading to hyperglycemia. It is well known that resistance training combats metabolic dysfunction in obese patients with type 2 diabetes mellitus by increasing insulin sensitivity. Resistance training also has evidence of protective effects against type 2 diabetes mellitus. Obese patients (≥30 BMI) who engaged in 150 minutes or more of resistance training per week had an estimated 60% reduction in risk of developing type 2 diabetes mellitus compared to their peers who engaged in less resistance training (Strasser, 2013).
This is important in the context of mental health treatment as many of our pharmacologic therapies are associated with metabolic syndrome and exercise can be used as a tool to help prevent and/or treat any adverse metabolic effects.
Sexual Health & Testosterone Therapy
Sexual function and dysfunction are common concerns amongst patients with depression and those being treated with SSRIs. Reviews of the literature for Episode 96 showed that physical activity can improve sexual health. Specifically, resistance training has a powerful association with increased testosterone and improved sexual function. Its effect on sexual health and performance was demonstrated recently in a 2017 meta-analysis on patients with prostate cancer undergoing androgen deprivation therapy. Groups using resistance training showed less decline in sexual desire and erectile dysfunction compared to control groups (Yunfeng, 2017).
Another study showed an association between fitness and sexual health in women, with sexual arousal being predicted by levels of cardiovascular endurance (Jiannie, 2018). A study in the International Journal of Sports Medicine demonstrated that one strength training workout can raise testosterone in young adults and elderly adults with (p < 0.5) in the young adults and no significant differences between the groups (Smilios, 2006).
A 2019 position statement affirmed that testosterone therapy has a positive effect on sexual desire in women with hypoactive sexual desire and dysfunction and recommends treatment for 3 to 6 months with frequent monitoring to avoid supraphysiologic concentrations (Weiss et al., 2019). A review from Bolour et al. (2005) found that postmenopausal women with loss of libido that were given low-dose testosterone in addition to estrogen significantly improved multiple facets of sexual functioning, including libido and sexual desire, arousal, frequency and satisfaction. They also found that the addition of androgens improved sense of well-being and other psychological factors. One study of transdermal testosterone therapy showed improved scores of anxiety, depressed mood, positive well-being, self-confidence, general health and vitality, and aspects of sexuality (Goldstat et al., 2003).
The APHRODITE trial investigated the effects of transdermal testosterone patches in postmenopausal women not using concomitant estrogen and found improvement in sexual function with minimal androgenic side effects of mildly increased hair growth with the higher 300 μg dose (Davis et al., 2008). Reviews of safety of testosterone therapy in women suggest that 150 μg or 300 μg of transdermal testosterone per day appears to be the safest method of administration (Al-Imari, 2012).
A more recent systematic review and meta-analysis Islam et al. (2019) (36 RCTs, n= 8,480 participants) receiving >12 weeks of testosterone therapy. No restriction was placed on type of menopause (natural or surgical) or use of concurrent hormone treatment (estrogen with or without progesterone). They found that testosterone significantly increased sexual function (e.g. sexual event frequency, sexual desire, pleasure, arousal, self-image) in postmenopausal women. Oral administration of testosterone showed increases in LDL-cholesterol and reductions in total cholesterol, HDL-cholesterol, and triglycerides, which was not seen when administered transdermally. Testosterone was associated with a significantly greater likelihood of reporting acne and hair growth and overall increase in weight, but no serious adverse events were recorded.
Studies have found that testosterone is necessary in maintaining a functional contractile and relaxant machinery, essential in the underlying mechanism of the peripheral arousal response (Cipriani et al., 2022). Women with female sexual dysfunction (n=81) were recruited to assess the effects of 6-month systemic testosterone (T) administration on clitoral color doppler ultrasound. The four different treatment groups were (1) local non-hormonal moisturizer, (2) transdermal 2% T gel 300 mcg/day (T group), (3) local estrogen (E group), and (4) combined therapy (T+E group). Testosterone therapy significantly increased clitoral artery peak systolic velocity when compared to both non-hormonal and estrogen groups. T treatment was associated with significantly higher Female Sexual Function Index desire, pain, arousal, lubrication, orgasm, and total scores at 6-months visit vs. baseline. Similar findings were observed in T + E group. T administration alone and combined with local estrogens was associated with a positive effect on clitoral blood flow and a clinical improvement in sexual function.
References:
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., Kim, J. S., Heo, S., Alves, H., White, S. M., Wojcicki, T. R., Mailey, E., Vieira, V. J., Martin, S. A., Pence, B. D., Woods, J. A., McAuley, E., & Kramer, A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. https://doi.org/10.1073/pnas.1015950108
Oudbier, S. J., Goh, J., Looijaard, S. M. L. M., Reijnierse, E. M., Meskers, C. G. M., & Maier, A. B. (2022). Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function. The journals of gerontology. Series A, Biological sciences and medical sciences, 77(10), 1959–1968. https://doi.org/10.1093/gerona/glac121
Marinus, N., Hansen, D., Feys, P., Meesen, R., Timmermans, A., & Spildooren, J. (2019). The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sports medicine (Auckland, N.Z.), 49(10), 1529–1546. https://doi.org/10.1007/s40279-019-01148-z
De Sousa, R. A. L., Rocha-Dias, I., de Oliveira, L. R. S., Improta-Caria, A. C., Monteiro-Junior, R. S., & Cassilhas, R. C. (2021). Molecular mechanisms of physical exercise on depression in the elderly: a systematic review. Molecular Biology Reports, 48(4), 3853-3862.
Wu, J., Xiao, W., Yip, J., Peng, L., Zheng, K., Takyi Bentil, O., & Ren, Z. (2022). Effects of Exercise on Neural Changes in Inhibitory Control: An ALE Meta-Analysis of fMRI Studies. Frontiers in human neuroscience, 16, 891095. https://doi.org/10.3389/fnhum.2022.891095
Huang, X., Ma, J., Ying, Y., Liu, K., Jing, C., & Hao, G. (2021). The handgrip strength and risk of depressive symptoms: a meta-analysis of prospective cohort studies. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation, 30(9), 2467–2474. https://doi.org/10.1007/s11136-021-02858-6
Cabanas-Sánchez, V., Esteban-Cornejo, I., Parra-Soto, S., Petermann-Rocha, F., Gray, S. R., Rodríguez-Artalejo, F., Ho, F. K., Pell, J. P., Martínez-Gómez, D., & Celis-Morales, C. (2022). Muscle strength and incidence of depression and anxiety: findings from the UK Biobank prospective cohort study. Journal of cachexia, sarcopenia and muscle, 13(4), 1983–1994. https://doi.org/10.1002/jcsm.12963
Muhammad, T., & Maurya, P. (2022). Relationship between handgrip strength, depression and cognitive functioning among older adults: Evidence from longitudinal ageing study in India. International journal of geriatric psychiatry, 37(8), 10.1002/gps.5776. https://doi.org/10.1002/gps.5776
Mahmoudi, A., Amirshaghaghi, F., Aminzadeh, R., & Mohamadi Turkmani, E. (2022). Effect of Aerobic, Resistance, and Combined Exercise Training on Depressive Symptoms, Quality of Life, and Muscle Strength in Healthy Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biological Research For Nursing, 10998004221104850.
Marques, A., Gomez-Baya, D., Peralta, M., Frasquilho, D., Santos, T., Martins, J., ... & Gaspar de Matos, M. (2020). The effect of muscular strength on depression symptoms in adults: a systematic review and meta-analysis. International journal of environmental research and public health, 17(16), 5674.
Marques, A., Bordado, J., Peralta, M., Gouveia, E. R., Tesler, R., Demetriou, Y., & Gomez Baya, D. (2020). Cross-sectional and prospective relationship between physical activity and depression symptoms. Scientific reports, 10(1), 16114. https://doi.org/10.1038/s41598-020-72987-4
Yu, Q., Wong, K. K., Lei, O. K., Nie, J., Shi, Q., Zou, L., & Kong, Z. (2022). Comparative Effectiveness of Multiple Exercise Interventions in the Treatment of Mental Health Disorders: A Systematic Review and Network Meta-Analysis. Sports medicine - open, 8(1), 135. https://doi.org/10.1186/s40798-022-00529-5
Heissel, A., Heinen, D., Brokmeier, L. L., Skarabis, N., Kangas, M., Vancampfort, D., Stubbs, B., Firth, J., Ward, P. B., Rosenbaum, S., Hallgren, M., & Schuch, F. (2023). Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression. British journal of sports medicine, bjsports-2022-106282. Advance online publication. https://doi.org/10.1136/bjsports-2022-106282
Singh, B., Olds, T., Curtis, R., Dumuid, D., Virgara, R., Watson, A., Szeto, K., O'Connor, E., Ferguson, T., Eglitis, E., Miatke, A., Simpson, C. E., & Maher, C. (2023). Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. British journal of sports medicine, bjsports-2022-106195. Advance online publication. https://doi.org/10.1136/bjsports-2022-106195
Gordon, B. R., McDowell, C. P., Hallgren, M., Meyer, J. D., Lyons, M., & Herring, M. P. (2018). Association of Efficacy of Resistance Exercise Training With Depressive Symptoms: Meta-analysis and Meta-regression Analysis of Randomized Clinical Trials. JAMA psychiatry, 75(6), 566–576. https://doi.org/10.1001/jamapsychiatry.2018.0572
Nebiker, L., Lichtenstein, E., Minghetti, A., Zahner, L., Gerber, M., Faude, O., & Donath, L. (2018). Moderating Effects of Exercise Duration and Intensity in Neuromuscular vs. Endurance Exercise Interventions for the Treatment of Depression: A Meta-Analytical Review. Frontiers in psychiatry, 9, 305. https://do.org/10.3389/fpsyt.2018.00305
Nasstasia, Y., Baker, A. L., Lewin, T. J., Halpin, S. A., Hides, L., Kelly, B. J., & Callister, R. (2019). Differential treatment effects of an integrated motivational interviewing and exercise intervention on depressive symptom profiles and associated factors: A randomised controlled cross-over trial among youth with major depression. Journal of affective disorders, 259, 413–423. https://doi.org/10.1016/j.jad.2019.08.035
Bennie, J. A., Teychenne, M. J., De Cocker, K., & Biddle, S. J. H. (2019). Associations between aerobic and muscle-strengthening exercise with depressive symptom severity among 17,839 U.S. adults. Preventive medicine, 121, 121–127. https://doi.org/10.1016/j.ypmed.2019.02.022
Oftedal, S., Smith, J., Vandelanotte, C., Burton, N. W., & Duncan, M. J. (2019). Resistance training in addition to aerobic activity is associated with lower likelihood of depression and comorbid depression and anxiety symptoms: A cross sectional analysis of Australian women. Preventive medicine, 126, 105773. https://doi.org/10.1016/j.ypmed.2019.105773
Plag, J., Schmidt-Hellinger, P., Klippstein, T., Mumm, J. L. M., Wolfarth, B., Petzold, M. B., & Ströhle, A. (2020). Working out the worries: A randomized controlled trial of high intensity interval training in generalized anxiety disorder. Journal of anxiety disorders, 76, 102311. https://doi.org/10.1016/j.janxdis.2020.102311
Aylett, E., Small, N., & Bower, P. (2018). Exercise in the treatment of clinical anxiety in general practice - a systematic review and meta-analysis. BMC health services research, 18(1), 559. https://doi.org/10.1186/s12913-018-3313-5
Schoenfeld, B. J., Grgic, J., Ogborn, D., & Krieger, J. W. (2017). Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. Journal of strength and conditioning research, 31(12), 3508–3523. https://doi.org/10.1519/JSC.0000000000002200
Moraes, H. S., Set al.. (2020). Is Strength Training as Effective as Aerobic Training for Depression in Older Adults? A Randomized Controlled Trial. Neuropsychobiology, 79(2), 141–149. https://doi.org/10.1159/000503750
Barahona-Fuentes, G., Huerta Ojeda, Á., & Chirosa-Ríos, L. (2021). Effects of Training with Different Modes of Strength Intervention on Psychosocial Disorders in Adolescents: A Systematic Review and Meta-Analysis. International journal of environmental research and public health, 18(18), 9477. https://doi.org/10.3390/ijerph18189477
Neumann, R. J., Ahrens, K. F., Kollmann, B., Goldbach, N., Chmitorz, A., Weichert, D., Fiebach, C. J., Wessa, M., Kalisch, R., Lieb, K., Tüscher, O., Plichta, M. M., Reif, A., & Matura, S. (2022). The impact of physical fitness on resilience to modern life stress and the mediating role of general self-efficacy. European archives of psychiatry and clinical neuroscience, 272(4), 679–692. https://doi.org/10.1007/s00406-021-01338-9
Szuhany, K. L., Malgaroli, M., & Bonanno, G. A. (2023). Physical activity may buffer against depression and promote resilience after major life stressors. Mental health and physical activity, 24, 100505. https://doi.org/10.1016/j.mhpa.2023.100505
Moya, N. A., Tanner, M. K., Smith, A. M., Balolia, A., Davis, J. K. P., Bonar, K., Jaime, J., Hubert, T., Silva, J., Whitworth, W., Loetz, E. C., Bland, S. T., & Greenwood, B. N. (2020). Acute exercise enhances fear extinction through a mechanism involving central mTOR signaling. Neurobiology of learning and memory, 176, 107328. https://doi.org/10.1016/j.nlm.2020.107328
Tanner, M. K., Hake, H. S., Bouchet, C. A., & Greenwood, B. N. (2018). Running from fear: Exercise modulation of fear extinction. Neurobiology of learning and memory, 151, 28–34. https://doi.org/10.1016/j.nlm.2018.03.021
Powers, M. B., Medina, J. L., Burns, S., Kauffman, B. Y., Monfils, M., Asmundson, G. J., Diamond, A., McIntyre, C., & Smits, J. A. (2015). Exercise Augmentation of Exposure Therapy for PTSD: Rationale and Pilot Efficacy Data. Cognitive behaviour therapy, 44(4), 314–327. https://doi.org/10.1080/16506073.2015.1012740
Jadhakhan, F., Lambert, N., Middlebrook, N., Evans, D. W., & Falla, D. (2022). Is exercise/physical activity effective at reducing symptoms of post-traumatic stress disorder in adults - A systematic review. Frontiers in psychology, 13, 943479. https://doi.org/10.3389/fpsyg.2022.943479
Reis, D. J., Gaddy, M. A., & Chen, G. J. (2022). Exercise to Reduce Posttraumatic Stress Disorder Symptoms in Veterans. Federal practitioner : for the health care professionals of the VA, DoD, and PHS, 39(4), 158–166. https://doi.org/10.12788/fp.0248
Hegberg, N. J., Hayes, J. P., & Hayes, S. M. (2019). Exercise Intervention in PTSD: A Narrative Review and Rationale for Implementation. Frontiers in psychiatry, 10, 133. https://doi.org/10.3389/fpsyt.2019.00133
LeardMann, C. A., Kelton, M. L., Smith, B., Littman, A. J., Boyko, E. J., Wells, T. S., Smith, T. C., & Millennium Cohort Study Team (2011). Prospectively assessed posttraumatic stress disorder and associated physical activity. Public health reports (Washington, D.C. : 1974), 126(3), 371–383. https://doi.org/10.1177/003335491112600311
Rosenbaum, S., Sherrington, C., & Tiedemann, A. (2015). Exercise augmentation compared with usual care for post-traumatic stress disorder: a randomized controlled trial. Acta psychiatrica Scandinavica, 131(5), 350–359. https://doi.org/10.1111/acps.12371
Zika, M. A., & Becker, L. (2021). Physical Activity as a Treatment for Social Anxiety in Clinical and Non-clinical Populations: A Systematic Review and Three Meta-Analyses for Different Study Designs. Frontiers in human neuroscience, 15, 653108. https://doi.org/10.3389/fnhum.2021.653108
Dong, Z., Wang, P., Xin, X., Li, S., Wang, J., Zhao, J., & Wang, X. (2022). The relationship between physical activity and trait anxiety in college students: The mediating role of executive function. Frontiers in human neuroscience, 16, 1009540. https://doi.org/10.3389/fnhum.2022.1009540
Bredin, S. S. D., Kaufman, K. L., Chow, M. I., Lang, D. J., Wu, N., Kim, D. D., & Warburton, D. E. R. (2022). Effects of Aerobic, Resistance, and Combined Exercise Training on Psychiatric Symptom Severity and Related Health Measures in Adults Living With Schizophrenia: A Systematic Review and Meta-Analysis. Frontiers in cardiovascular medicine, 8, 753117. https://doi.org/10.3389/fcvm.2021.753117
Girdler, S. J., Confino, J. E., & Woesner, M. E. (2019). Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacology bulletin, 49(1), 56–69.
Pajonk, F. G., Wobrock, T., Gruber, O., Scherk, H., Berner, D., Kaizl, I., Kierer, A., Müller, S., Oest, M., Meyer, T., Backens, M., Schneider-Axmann, T., Thornton, A. E., Honer, W. G., & Falkai, P. (2010). Hippocampal plasticity in response to exercise in schizophrenia. Archives of general psychiatry, 67(2), 133–143. https://doi.org/10.1001/archgenpsychiatry.2009.193
Kim, H. J., Song, B. K., So, B., Lee, O., Song, W., & Kim, Y. (2014). Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: a pilot study. Psychiatry research, 220(3), 792–796. https://doi.org/10.1016/j.psychres.2014.09.020
Dauwan, M., Begemann, M. J., Heringa, S. M., & Sommer, I. E. (2016). Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophrenia bulletin, 42(3), 588–599. https://doi.org/10.1093/schbul/sbv164
Firth, J., Stubbs, B., Rosenbaum, S., Vancampfort, D., Malchow, B., Schuch, F., Elliott, R., Nuechterlein, K. H., & Yung, A. R. (2017). Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophrenia bulletin, 43(3), 546–556. https://doi.org/10.1093/schbul/sbw115
Keller-Varady, K., Varady, P. A., Röh, A., Schmitt, A., Falkai, P., Hasan, A., & Malchow, B. (2018). A systematic review of trials investigating strength training in schizophrenia spectrum disorders. Schizophrenia research, 192, 64–68. https://doi.org/10.1016/j.schres.2017.06.008
Silva, B. A., Cassilhas, R. C., Attux, C., Cordeiro, Q., Gadelha, A. L., Telles, B. A., Bressan, R. A., Ferreira, F. N., Rodstein, P. H., Daltio, C. S., Tufik, S., & de Mello, M. T. (2015). A 20-week program of resistance or concurrent exercise improves symptoms of schizophrenia: results of a blind, randomized controlled trial. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999), 37(4), 271–279. https://doi.org/10.1590/1516-4446-2014-1595
Heggelund, J., Morken, G., Helgerud, J., Nilsberg, G. E., & Hoff, J. (2012). Therapeutic effects of maximal strength training on walking efficiency in patients with schizophrenia - a pilot study. BMC research notes, 5, 344. https://doi.org/10.1186/1756-0500-5-344
Subotnik, K. L., McEwen, S. C., Ventura, J., Turner, L. R., Sturdevant, Y., Niess, T. L., Casaus, L. R., Distler, M. G., Zito, M. F., Hellemann, G. S., Nguyen, C. D., & Nuechterlein, K. H. (2023). Exercise Predicts a Good Night's Sleep: Preliminary Findings from a UCLA Study of First-Episode Schizophrenia. Behavioral sciences (Basel, Switzerland), 13(2), 88. https://doi.org/10.3390/bs13020088
Liang, X., Li, R., Wong, S. H. S., Sum, R. K. W., & Sit, C. H. P. (2021). The impact of exercise interventions concerning executive functions of children and adolescents with attention-deficit/hyperactive disorder: a systematic review and meta-analysis. The international journal of behavioral nutrition and physical activity, 18(1), 68. https://doi.org/10.1186/s12966-021-01135-6
Kadri, A., Slimani, M., Bragazzi, N. L., Tod, D., & Azaiez, F. (2019). Effect of Taekwondo Practice on Cognitive Function in Adolescents with Attention Deficit Hyperactivity Disorder. International journal of environmental research and public health, 16(2), 204. https://doi.org/10.3390/ijerph16020204
Yang, W., Liang, X., & Sit, C. H. (2022). Physical activity and mental health in children and adolescents with intellectual disabilities: a meta-analysis using the RE-AIM framework. The international journal of behavioral nutrition and physical activity, 19(1), 80. https://doi.org/10.1186/s12966-022-01312-1
Giménez-Meseguer, J., Tortosa-Martínez, J., & Cortell-Tormo, J. M. (2020). The Benefits of Physical Exercise on Mental Disorders and Quality of Life in Substance Use Disorders Patients. Systematic Review and Meta-Analysis. International journal of environmental research and public health, 17(10), 3680. https://doi.org/10.3390/ijerph17103680
Aasa, U., Svartholm, I., Andersson, F., & Berglund, L. (2017). Injuries among weightlifters and powerlifters: a systematic review. British journal of sports medicine, 51(4), 211–219. https://doi.org/10.1136/bjsports-2016-096037
Pillinger, T., McCutcheon, R. A., Vano, L., Mizuno, Y., Arumuham, A., Hindley, G., Beck, K., Natesan, S., Efthimiou, O., Cipriani, A., & Howes, O. D. (2020). Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. The lancet. Psychiatry, 7(1), 64–77. https://doi.org/10.1016/S2215-0366(19)30416-X
Marzolini, S., Oh, P. I., & Brooks, D. (2012). Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: a meta-analysis. European journal of preventive cardiology, 19(1), 81–94. https://doi.org/10.1177/1741826710393197
Strasser, B., & Pesta, D. (2013). Resistance training for diabetes prevention and therapy: experimental findings and molecular mechanisms. BioMed research international, 2013, 805217. https://doi.org/10.1155/2013/805217
Mehren, A., Reichert, M., Coghill, D., Müller, H. H. O., Braun, N., & Philipsen, A. (2020). Physical exercise in attention deficit hyperactivity disorder - evidence and implications for the treatment of borderline personality disorder. Borderline personality disorder and emotion dysregulation, 7, 1. https://doi.org/10.1186/s40479-019-0115-2
St-Amour, S., Cailhol, L., Ruocco, A. C., & Bernard, P. (2022). Acute Effect of Physical Exercise on Negative Affect in Borderline Personality Disorder: A Pilot Study. Clinical psychology in Europe, 4(2), e7495. https://doi.org/10.32872/cpe.7495
Liang, X., Li, R., Wong, S. H. S., Sum, R. K. W., Wang, P., Yang, B., & Sit, C. H. P. (2022). The Effects of Exercise Interventions on Executive Functions in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Sports medicine (Auckland, N.Z.), 52(1), 75–88. https://doi.org/10.1007/s40279-021-01545-3
Fabiano, N., Gupta, A., Fiedorowicz, J. G., Firth, J., Stubbs, B., Vancampfort, D., Schuch, F. B., Carr, L. J., & Solmi, M. (2023). The effect of exercise on suicidal ideation and behaviors: A systematic review and meta-analysis of randomized controlled trials. Journal of affective disorders, 330, 355–366. https://doi.org/10.1016/j.jad.2023.02.071
Yunfeng, G., Weiyang, H., Xueyang, H., Yilong, H., & Xin, G. (2017). Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: An update meta-analysis. Medicine, 96(27), e7368. https://doi.org/10.1097/MD.0000000000007368
Jiannine L. M. (2018). An investigation of the relationship between physical fitness, self-concept, and sexual functioning. Journal of education and health promotion, 7, 57. https://doi.org/10.4103/jehp.jehp_157_17
Smilios, I., Pilianidis, T., Karamouzis, M., Parlavantzas, A., & Tokmakidis, S. P. (2007). Hormonal responses after a strength endurance resistance exercise protocol in young and elderly males. International journal of sports medicine, 28(5), 401–406. https://doi.org/10.1055/s-2006-924366
Weiss, R. V., Hohl, A., Athayde, A., Pardini, D., Gomes, L., Oliveira, M., Meirelles, R., Clapauch, R., & Spritzer, P. M. (2019). Testosterone therapy for women with low sexual desire: a position statement from the Brazilian Society of Endocrinology and Metabolism. Archives of endocrinology and metabolism, 63(3), 190–198. https://doi.org/10.20945/2359-3997000000152
Cipriani, S., Maseroli, E., Di Stasi, V., Scavello, I., Todisco, T., Rastrelli, G., Fambrini, M., Sorbi, F., Petraglia, F., Jannini, E. A., Maggi, M., & Vignozzi, L. (2021). Effects of testosterone treatment on clitoral haemodynamics in women with sexual dysfunction. Journal of endocrinological investigation, 44(12), 2765–2776. https://doi.org/10.1007/s40618-021-01598-1
Islam, R. M., Bell, R. J., Green, S., Page, M. J., & Davis, S. R. (2019). Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. The lancet. Diabetes & endocrinology, 7(10), 754–766. https://doi.org/10.1016/S2213-8587(19)30189-5
Al-Imari, L., & Wolfman, W. L. (2012). The safety of testosterone therapy in women. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstétrique et gynécologie du Canada : JOGC, 34(9), 859–865. https://doi.org/10.1016/S1701-2163(16)35385-3
Davis, S. R., Moreau, M., Kroll, R., Bouchard, C., Panay, N., Gass, M., Braunstein, G. D., Hirschberg, A. L., Rodenberg, C., Pack, S., Koch, H., Moufarege, A., Studd, J., & APHRODITE Study Team (2008). Testosterone for low libido in postmenopausal women not taking estrogen. The New England journal of medicine, 359(19), 2005–2017. https://doi.org/10.1056/NEJMoa0707302
Davis, S. R., Moreau, M., Kroll, R., Bouchard, C., Panay, N., Gass, M., Braunstein, G. D., Hirschberg, A. L., Rodenberg, C., Pack, S., Koch, H., Moufarege, A., Studd, J., & APHRODITE Study Team (2008). Testosterone for low libido in postmenopausal women not taking estrogen. The New England journal of medicine, 359(19), 2005–2017. https://doi.org/10.1056/NEJMoa0707302