Episode 221: Sauna & Heat Exposure’s Impact on Mental & Physical Health
By listening to this episode, you can earn 1.5 Psychiatry CME Credits.
Other Places to listen: iTunes, Spotify
Article Authors: Liam Browning, David Puder, MD
A Global History Of Heat Therapy: From Ancient Finnish Saunas to Modern Medicinal Uses
Timeline of Heat Therapy: A Global History
Circa 4000-2000 B.C.E.:
Ancient Finnish Saunas: Early saunas originated in Finland as large pits lined with animal skins, where fires were built to heat stones. These saunas were used for both medicinal and social purposes.
Circa 2000-1000 B.C.E.:
Ancient Egyptian Sand Baths: Egyptians used hot sand baths for treating inflammation and pain, representing early passive hyperthermia therapy.
Circa 1000 B.C.E.:
Traditional Chinese Medicine and Ayurveda: In China and India, heat therapy techniques like moxibustion and steam treatments are employed for improving circulation, reducing pain, and promoting detoxification.
Circa 500 B.C.E.-500 C.E:
Greek and Roman Bathhouses: The Greeks began using heated baths and steam baths, referred to as laconica. Communal bathhouses became central in Roman society, with figures like Galen writing about hot baths to treat melancholia and other ailments.
Ottoman Hammams: Public baths in the Middle East and North Africa, known as hammams, were common throughout the region, offering both hygienic and therapeutic benefits. It later rose to greater prominence as an integral part of Islamic culture beginning in the 7th-8th century. The buildings contained a hot room (Hararet) with underfloor heating by a furnace and also a cold room (Soğukluk) for cold water immersion.
Native American Sweat Lodges: Indigenous tribes across North America developed sweat lodges, used for purification, spiritual rituals, and healing. These lodges were a staple in Native American culture and closely mirror sauna practices.
Middle Ages (Circa 500-1500 C.E.):
European Bathhouses: Communal bathhouses were widespread across medieval Europe, combining hygiene with socializing and therapeutic practices.
Mayan Temazcal: In Mesoamerica, the temazcal, a type of sweat lodge, was used for ritualistic cleansing and healing, particularly in the context of childbirth and illness.
19th Century C.E.:
Victorian-Era Turkish Baths (Hammams): Western Europe, particularly the U.K., experienced a revival in communal heat therapy with the introduction of hammams, or Turkish baths.
1900s C.E.:
“Malaria Fever Cure”: Austrian physicians Julius Wagner-Jauregg and Rudolf Rosenblum introduced heat therapy via induced fever to treat psychiatric symptoms related to syphilis, particularly neurosyphilis, and later experimented with it for other mental health disorders. Before the discovery of penicillin, this method led to significant improvement in about 30-50% of cases; however, some patients experienced worsening symptoms, and approximately 15% died from complications.
1960s C.E.:
Development of Infrared Saunas: Infrared saunas, which heat the body directly using radiant heat, were introduced as a modern alternative to traditional saunas, allowing for lower ambient temperatures.
21st Century C.E.:
Modern Medical Hyperthermia: Hyperthermia treatments in oncology emerged, where heat is used to target and destroy cancer cells as part of therapeutic protocols.
Present Day:
Sauna Culture in Finland: With an estimated 3.2 million saunas for a population of 5.5 million, sauna use remains deeply embedded in Finnish culture and is recognized for its cardiovascular and wellness benefits globally.
How Physical Health Conditions Elevate Depression Risk: Unveiling The Mind-Body Connection
There is a strong link between physical health and mental health.
Increased risk of depression with these diseases:
Key Findings From The Kuopio Ischemic Heart Disease Study: The Long-Term Benefits Of Sauna Use
The strongest literature on sauna is from a longitudinal observational study in Finland, called the Kuopio Ischemic Heart Disease (KIHD) Risk Factor Study.
2,327 middle-aged men (mean age 53 y/o)
Grouped participants by sauna frequency:
Group 1: Once per week
Group 2: 2-3 times per week
Group 3: 4-7 times per week
Most participants used a dry sauna at an average temperature of 80°C (170°F) for an average of 14 minutes (range 2-90 minutes)
One of the first publications of this study looked at cardiovascular outcomes, stroke, and all-cause mortality after 21 years (Laukkanen et al., 2015).
Compared to once weekly sauna users, the 3-7x/week users had a 40% reduced risk of all-cause mortality, 50% reduced risk of fatal cardiovascular disease, 48% risk of fatal coronary artery disease, and a 63% decreased risk of sudden cardiac death.
This is after adjusting for age, BMI, SBP, LDL, smoking, previous MI, T2DM, activity, and SES.
In the unadjusted analyses, the 2-3x/group had a significantly reduced risk of these outcomes, suggesting a dose-response relationship.
The dose-response effect was also seen with sauna session duration.
Compared to sauna users with sessions less than 11 minutes, the HR for sudden cardiac death was 0.93 (95% CI, 0.67-1.28) and 0.48 (95% CI, 0.31-0.75) for sauna users with average sessions of 11-19 minutes and >19 minutes respectively, supporting this dose-response relationship.
Follow-up studies from this same cohort showed that compared to once weekly users and controlling for similar confounds, the 4-7x/week users had:
47% lower risk of hypertension (Zaccardi et al., 2017)
62% lower risk of stroke (Kunutsor et al., 2018)
66% decreased risk of dementia and a 65% lower risk of Alzheimer's disease (Laukkanen et al., 2017)
37% reduced risk of pneumonia (Kunutsor et al., 2017)
78% reduced risk of having a psychotic disorder diagnosis on Finnish Hospital Registry (Laukkanen et al., 2019)
This did not change when considering sauna session duration
Reported rate of psychosis per 1,000 person years
Included delirium psychosis potentially
At the beginning of the study they excluded study participants who were on antipsychotic medications.
Sauna vs. Other Interventions: Comparing Long-Term Health Impacts Of Exercise, Diet, and More
Compared to other interventions
Aerobic exercise
35% reduction in all-cause mortality in participants of the KIHD cohort who were above the sample median VO2 max value compared to the participant below the median (Kunutsor et al., 2017)
A meta-analysis of 11 prospective cohort studies by (Garcia et al., 2023) showed that exercising an equivalence of 8.75 metabolic equivalents (METs) (walking 2.5 hr/week) and 17.5 METS (walking 5hr/week) compared to 0 physical activity led to:
31% and 34% decrease of all-cause mortality respectively
29% and 35% CVD mortality
15% and 18% cancer mortality
Given the median in the study sample was 10.5 MET, this is relatively mild-moderate physical activity. The dose response seems to continue with vigorous physical activity according to other studies.
All-cause mortality by VO2 max percentile compared to the bottom 25th percentile (“Low”) (Mandsager et al., 2018):
25-50th percentile (“Below Average): 20% reduced risk
50-75th percentile (“Above Average”): 63% reduced risk
75-97.5th percentile (“High”): 74% reduced risk
97.5-100th percentile (“Elite”): 80% reduced risk
Even going from “Below Average” to “Above Average” conferred 29% reduced risk
Diet
Greater adherence to mediterranean diet compared to lowest adherence to mediterranean diet according to a meta-analysis of 43 studies (Becerra-Tomás et al., 2020)
21% reduced risk of CVD mortality
20% reduced risk of stroke
Meta-analysis of 17 prospective studies showed that being in the top quintile of EPA/DHA omega 3s (marine sources) was associated with a 15-18% reduced all-cause mortality risk compared to bottom quintile (Harris et al., 2021).
Limitations/cautions of the KIHD study
Differences in baseline characteristics between once weekly users and 4-7x/week users (although this was controlled)
4-7x/week group was 2 years younger than once weekly group
Less smokers (20% vs. 36%)
More prevalent alcohol consumption (95% vs. 83.%)
Less diabetes (2% vs. 6.6%)
Less hypertension (28.1% vs. 35%)
Healthy user bias
Somewhat mitigated SES differences given how prevalent sauna usage is in Finland
Takeaways
Regardless of subtle baseline differences between the groups, the results are striking.
20-minute dry sauna sessions at least 4x/week and 80°C (172°F) show the greatest benefit.
However, hot baths may be comparable if a sauna is unavailable, as one prospective cohort study in Japan showed reduced risk of stroke and cardiovascular events with daily hot tub baths:
Ukai et al. (2020) https://pubmed.ncbi.nlm.nih.gov/32209614/
Reduce the risk of adverse cardiovascular outcomes by about 23–46%.
Hot tub baths almost daily or every day versus zero to two times/week were associated with risk reductions in cardiovascular outcomes ranging from 23–46%.
However, this is in a younger cohort (40-59 y/o) and in Japan, where cardiovascular deaths are not as common at this age.
Cardiovascular Health Insights: Findings from the KIHD Study and Other Key Research on Physical Wellness
Blood pressure:
The KIHD study from Zaccardi et al., 2017 showed that sauna sessions 4–7 times weekly was associated with a 47% relative decrease in the likelihood of developing hypertension in the 25-year follow up, controlling for age, smoking, body mass index, glucose, creatinine, alcohol consumption, heart rate, family history of hypertension, socioeconomic status, and cardiorespiratory fitness. This effect was insignificant in 2-3 times/week when controlling for these confounds.
Sauna acutely decreases SBP/DBP by about 5-10mmHg for minutes to hours post-sauna (Laukkanen et al., 2018).
Pizzey et al., 2021 reviewed 4 RCTs of repeated (3-5d/week) immersion in hot water (38.5-40.5°C) showed an average reduction in SBP by 5.9 mmHg and DBP by 3.9 vs. sham control. This decrease was observed in healthy, obese, and heart-failure participants with hypertension.
Compare to SBP decreases observed with other lifestyle modifications:
1mmHg per kg of body weight loss in overweight individuals (Neter et al., 2003)
6-11mmHg with Dash Diet (emphasizes fruits, vegetables, whole grains, and low-fat dairy products while reducing sodium intake) (Sacks et al., 2001)
4-9mmHg with aerobic exercise (Cornelissen et al., 2005)
2-8 mmHg with sodium restriction (He & MacGregor, 2002)
2-4 mmHg with alcohol moderation (Xin et al., 2001)
And 10-15mg decreases in SBP with antihypertensive monotherapy (Paz et al., 2016)
The antihypertensive effects of sauna are thought to be due to endothelium-dependent dilatation, reduced arterial stiffness, modulation of the autonomic nervous system (Laukkanen et al., 2018).
Sauna may mimic exercise: The acute changes and after-effects of sauna on cardiac load, blood pressure, and vascular hemodynamics is extremely similar to that of moderate intensity exercise (Ketelhut & Ketelhut, 2019).
Sauna will frequently bring the heart rate to over 100 BPM and even to 150 BPM in more intense settings (Laukkanen & Kunutsor, 2024).
Does sauna provide a different benefit than exercise?
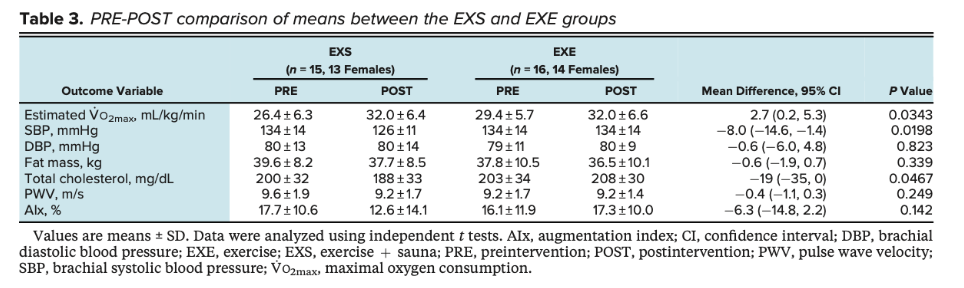
Lee et al., 2022 conducted a study comparing 8 weeks of exercise alone, exercise + sauna, and control participants. Both the exercise and sauna routines were progressive in nature. They found that, compared to exercise alone, exercise + sauna improved V02 max (2.7mL/kg/min), total cholesterol (-19mg/dL), and systolic bp (-8mmHg).
Limitations:
Sauna + exercise group experienced more time in which they had elevated HR. If sauna acts as an exercise mimic, time should be controlled.
Exercise group did not improve as expected.
Aerobic exercise in combination with frequent sauna use has a synergistic effect on lowering cardiovascular-related mortality and all-cause mortality, according to one article from the KIHD study. The strongest reductions in mortality were found in people with high cardiorespiratory fitness (>50th percentile VO2 max) and high frequent sauna bathing (>3 sessions/week) HR 0.60 (95% CI: 0.48–0.76), followed by high cardiorespiratory fitness and low frequent sauna bathing 0.63 (95% CI: 0.54–0.74), and then low cardiorespiratory fitness and high frequent sauna bathing 0.78 (95% CI: 0.64–0.96) (Kunutsor et al., 2017).
Median follow up 26.1 years
520 CVD deaths and 1124 all-cause death
DJP: different sports and how long people live, tennis seemed to win- 1) high SES or 2) interaction with people. How much of the sauna is interaction with people?
Exploring Additional Health Benefits of Heat Therapy: Insights into Heart Failure, Diabetes, Joint Disease, and More
A meta-analysis of low-quality studies involving heart failure patients suggests that heat therapy, such as sauna use, can help improve symptoms and reduce levels of B-type natriuretic peptide (BNP), a marker of heart failure severity (Ye et al., 2020).
Qualitative studies of Waon therapy, a far-infrared dry sauna treatment, show improvement in peripheral artery disease (PAD) through enhanced ankle-brachial index (ABI), increased blood flow, and the development of collateral vessels (Tei et al., 2007).
Type 2 diabetes
A review of five studies (Sebők et al., 2021) on Type 2 diabetes found that while there was no significant change in glucose or HbA1c levels with interventions like heat therapy, including sauna use, there was a consistent reduction in systolic blood pressure, averaging 5-10 mmHg. This improvement is important because sustained reductions in blood pressure are associated with a lower risk of cardiovascular events and complications in diabetic patients, making it a valuable adjunct in managing long-term cardiovascular health.
Inflammatory joint disease
Cozzi and colleagues (2018) reviewed several randomized-controlled trials (RCTs) that demonstrated the benefits of balneotherapy in improving the subjective clinical course of conditions like ankylosing spondylitis, enteropathic spondylitis, and psoriatic arthritis. The studies showed that patients experienced significant relief from symptoms such as pain, stiffness, and fatigue. These improvements were primarily attributed to the anti-inflammatory and pain-relieving effects of balneotherapy, which involves bathing in mineral-rich waters. The findings suggest that balneotherapy can be a valuable complementary treatment option for managing these chronic inflammatory conditions.
Sauna Use for Depression: An Evidence-Based Review Of Infrared And Whole-Body Hyperthermia Studies
The First RCT on Sauna Use for Depression: Masuda et al. (2005)
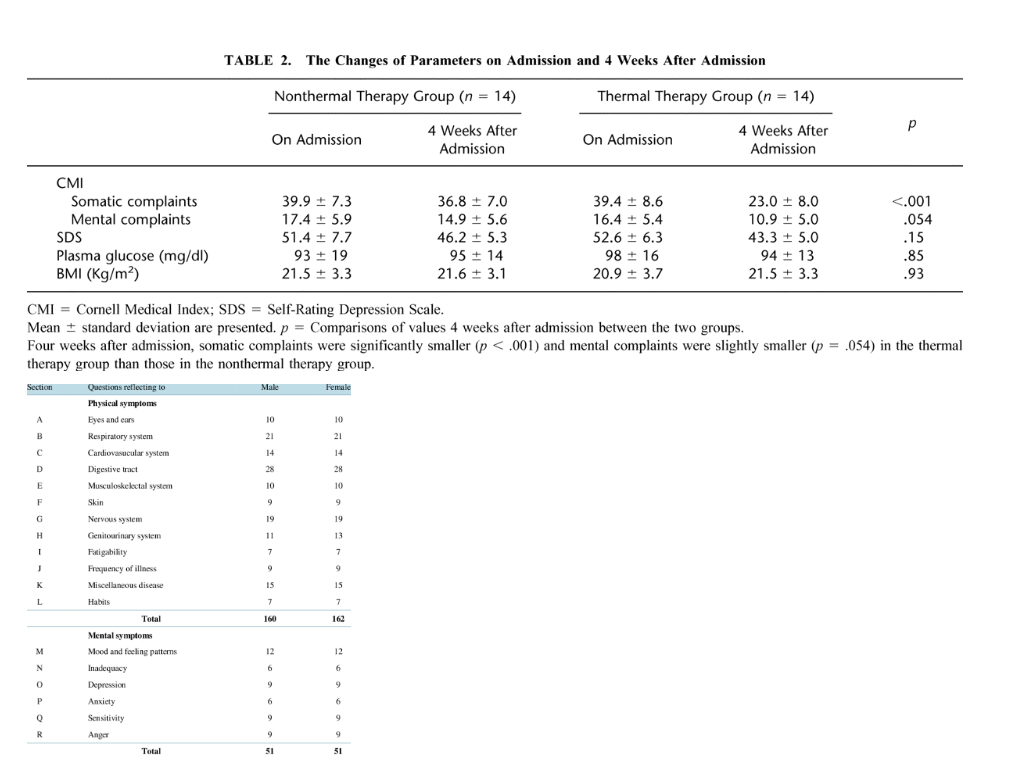
The only study on sauna use for depression was a randomized-controlled trial by Masuda et al. (2005).
28 individuals with mild depression and somatic symptoms were randomized to 4 weeks of infrared sauna sessions or bed rest.
Saunas were 5x/week at 60°C (140°F) for 15 minutes.
Somatic complaints were significantly decreased (t = 4.84, p < .001) and mental complaints were decreased to trend-level significance (t = 2.02, p = .054) in the sauna group according to the Cornell Medical Index.
There were no differences on the Self-rating Depression Scale.
Of note: this was not the most effective dose, as noted above, being of lower temperature and also lower duration of time.
Whole-Body Hyperthermia and Depression: A Review of Key Studies (2019)
Review of depression studies up until 2019: The impact of whole-body hyperthermia interventions on mood and depression – are we ready for recommendations for clinical application? (Hanusch & Jansenn, 2019)
Three studies provided whole-body heating in a hot bath and four in infrared chambers.
Infrared chambers
Most protocols included a 45-minute heating time to reach a rectal temperature of 38.5-39 °C followed 30-60 minutes of rest inside the chamber.
Bath
Water temperature of 37 °C (98.6 °F) and was increased by 1 °C every 10 min for 47 min on average (Hanusch & Jansenn, 2019)
Water temperature of 40.2 °C (104.3°F) for an average of 22.6 min (Naumann et al., 2017)
There were three open label studies and four RCTs
3/3 open label studies showed significant improvements in depressive symptoms. Two of the open label studies reported effect sizes: 1.71 and 1.85.
3/4 RCTs showed superiority for the hyperthermia group, with inconsistent effects.
Randomized-Controlled Trials on Whole-Body Hyperthermia for Depression
The most impressive of these RCTs was from Janssen et al. (2016): Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder A Randomized Clinical Trial
Methods
34 moderate to severely depressed patients randomized to whole-body hyperthermia (WBH) via infrared chamber (n=16) vs sham-controls who received very mild heat (n=14).
Participants underwent a single infrared session in which they sat in the heated chamber until their rectal temperature reached 38.6°C (101.5°F) (avg 47 min), followed by resting in the machine for 60 min.
Depressive symptoms were assessed 1, 2, 4, and 6 weeks after WBH using the Hamilton Rating Scale for Depression (HAMD).
Results
Active WBH group showed significantly reduced HAMD scores across the 6-week post intervention study period (WBH vs sham; week 1: −6.53, 95% CI, −9.90 to −3.16, P < .001, d = 2.23; week 2: −6.35, 95% CI, −9.95 to −2.74, P = .001, d = 2.11; week 4: −4.50, 95% CI, −8.17 to −0.84, P = .02, d = 1.66; and week 6: −4.27, 95% CI, −7.94 to −0.61, P = .02, d = 1.66).
Limitations/Takeaways
The treatment group did have a higher baseline expectancy of the treatment’s efficacy.
The authors did perform a moderation analysis controlling for expectancy effects, but did not include this variable in their original analysis. The effect size following the moderation analysis was also not reported.
However, it is worth noting 70% of the sham group did believe they received the active treatment.
Given this is a single session and is unlikely to provide much of a physiological stimulus lasting 6 weeks, more attention should have been given to the belief in treatment efficacy.
The other RCTs used multiple sessions:
Hüppe et al. (2009) randomized 36 patients to hyperthermia, sham-control, or waitlist control.
There were no significant differences in depressive symptoms, as baseline scores were low and different between groups.
Romeyke & Stummer (2014) allocated 104 patients with severe fibromyalgia and MDD to an average of 5 hyperthermia sessions or waitlist control.
Pain improved more in the hyperthermia group, but both groups improved in depressive symptoms, with no trend-level differences favoring the hyperthermia group (p = .055).
All patients received physical therapy, physiotherapy, acupuncture, psychotherapy.
Naumann et al. (2017) randomized 17 moderately depressed patients to 2 weekly hyperthermia baths at 40°C (104°F) for 22 minutes for 4 weeks vs. sham-control that received green light exposure.
Significantly more improvement in the hyperthermia group compared to sham-control at week 2 (p=.0370; d = .62 95% CI [-.05 to 1/29) but not at week 6.
General Takeaways and Limitations in Heat Therapy for Depression
Initial findings are promising, but there is a need for more, larger studies using repeated sauna sessions.
How Heat Exposure Enhances Antidepressant Effects: Unveiling The Science Behind Sauna Use and Mental Health
Heat stress and exercise increase the expression of brain-derived neurotrophic factor (BDNF) (Kojima et al., 2018)
Whole-body hyperthermia to a core body temperature of 39.5 °C (103.1 °F) via hot water bath (20-min at 42 °C [107.6 °F]) increased BDNF levels 66% for 15 minutes (Kojima et al., 2018).
Inflammation and Heat Exposure
Similar to exercise, whole body hyperthermia increases plasma levels of pro-inflammatory IL-6 and anti-inflammatory IL-10 (Katchinski et al., 1999; Windsor et al., 2018). However, the impact of these changes on depression and their potential to cause long-term alterations in inflammatory markers remains uncertain (Mac Giollabhui et al., 2024).
The Role of Heat Shock Proteins (HSPs) in Sauna Therapy: Mechanisms Linked to Physical and Therefore Antidepressant Effects
HSPs are a family of proteins observed in all forms of life from bacteria to humans and are crucial for a cell’s protein quality control systems. Cells are largely made of proteins, and over time, proteins will inevitably lose their function and shape. HSPs can physically change the shape of dysfunctional or misfolded proteins. They also help new proteins fold correctly, disaggregate proteins that are clumped together, and target proteins for degradation. When cells are faced with hypoxia, heat stress, nutritional stress, or other stressors, HSP are upregulated to help make new proteins and to repair damaged ones (Rosenzweig et al., 2019).
HSPs may be vital for preventing some of the effects of aging. The cell’s ability to counteract stress declines with age due to a variety of reasons that stem from metabolic dysfunction as a result of mitochondrial decline or accumulations of DNA mutations. Protein quality control systems also decline with age as well, as the number and efficiency of HSPs decreases. As a result, more defunct proteins are formed and our cells become less capable of handling them. Declines in particular HSPs are related to neurodegenerative disorders associated with protein aggregates such as Alzheimers, Parkinson’s, and Huntington disease, and may be related to normal cognitive decline. They also are important for recycling the proteins involved in neurotransmission and can be neuroprotective in cases of seizures and strokes (Stetler et al., 2010).
Sauna and exercise both increase the expression of HSPs, likely through the effects of hyperthermia at temperatures >38.5°C (101.3°F) (Hunt et al., 2019). To what extent elevated HSPs confer a benefit is unclear, but some passive heat exposure studies in rats and humans (serum) have observed that a 30-40% increase in HSP was associated with conserved muscle mass and greater mitochondrial function (Yoshihara et al., 2013; McGorm et al., 2018).
Hormonal Changes with Sauna Use: Short-Term Boosts in Growth Hormone, Testosterone, and Cortisol
Similar to aerobic or strength training, sauna increases growth hormone concentration in the short term, often by about 2- to 5-fold (Kukkonen-Harjula et al., 1989). Some studies have also reported small increases in testosterone while others have shown mixed effects on cortisol (Laukkanen & Kunutsor, 2024).
These effects seem similar to that of exercise, as they last only a few hours and do not seem additive when combined with exercise. For instance, one study did not observe an additional increase in GH, testosterone, or cortisol with the addition of sauna following endurance, strength, or a combined workout (Rissanen et al., 2020).
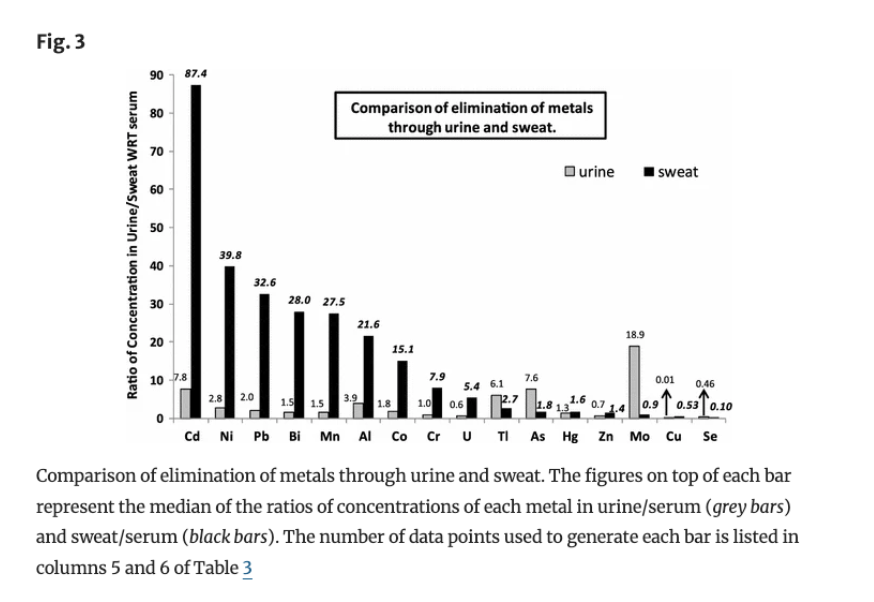
The Detoxifying Power of Sweating: How Sauna and Exercise Aid Heavy Metal Excretion
Sweating is often touted as a method to aid heavy metal detoxification. Research suggests that sweat can enhance the excretion of certain metals like aluminum (3.75-fold), cadmium (25-fold), cobalt (7-fold), and lead (17-fold) compared to urine (Genuis et al., 2011). However, the effectiveness and composition of sweat can differ based on whether it’s induced by passive hyperthermia (sauna) or active hyperthermia (exercise).
A small study (Kuan et al., 2022) found that sweat from exercise had higher concentrations of metals like nickel, lead, copper, and arsenic compared to sauna sweat, while mercury levels were similar. This difference likely stems from the greater metabolic activity during exercise, which mobilizes more toxins. In contrast, sauna-induced sweating primarily serves thermoregulation, potentially leading to lower concentrations of some metals.
There is evidence suggesting that dementia may be associated with elevated levels of certain heavy metals in the brain. For example, studies have identified higher concentrations of aluminum, mercury, and lead in individuals with Alzheimer’s disease, pointing to a potential link between metal accumulation and neurodegeneration. While cohort studies from Finland have shown a correlation between frequent sauna use and lower rates of dementia, it remains unclear whether this benefit is directly related to heavy metal excretion or other factors, such as improved cardiovascular health and reduced inflammation. It is important to note that the majority of heavy metals are excreted through stool, with smaller amounts eliminated via urine and sweat. In cases of significantly elevated heavy metal levels, chelation therapy remains the gold standard for treatment.
Safety And Precautions Of Heat Exposure: Risks, Contraindications, And Special Considerations
Risks in Extreme Competition:
Death and serious health issues can occur during high-intensity competitions in hot environments, especially when participants push their physical limits.
Pregnancy:
Exposure to extreme heat during pregnancy has been linked to central nervous system birth defects, such as anencephaly and spina bifida, and the suggested teratogenic threshold for core body temperature is 39.0 °C (102.2 °F) (Graham et al., 1998).
Contraindications:
Heat exposure should be avoided in individuals with certain conditions (Patrick & Johnson, 2021), including:
Alcohol use (due to impaired thermoregulation)
Hypotension (especially in older adults), which increases the risk of fainting
Recent myocardial infarction, unstable angina pectoris, and severe aortic stenosis
Altered or reduced sweat function, which impairs the body’s ability to regulate temperature
Hydration and Electrolyte Balance:
Dehydration is a common risk during heat exposure, which can lead to complications like heat stroke and fainting. Adequate water and electrolyte intake is essential, especially during prolonged sauna sessions or intense exercise.
Age-Related Risks:
Older adults are more susceptible to heat-related complications due to decreased cardiovascular resilience and sweat function. Additional caution is advised for this population.
Children are more vulnerable to heat exposure due to their underdeveloped thermoregulation, higher risk of dehydration, and reduced sweating efficiency. They are also less aware of their physical limits, increasing the risk of heat-related illnesses like heat exhaustion or heat stroke. For young children, especially under 6, sauna sessions should be brief (5-10 minutes) at lower temperatures, with close supervision and regular hydration. Gradual acclimatization and education on hydration and rest are key to minimizing risks.
Chronic Health Conditions:
Individuals with conditions such as diabetes, multiple sclerosis, or autonomic dysfunction may have reduced heat tolerance. Consultation with a healthcare provider is recommended before engaging in sauna use or intense heat exposure.
Acclimatization:
Gradually increasing exposure to heat, starting with shorter sessions at lower temperatures, can improve tolerance and reduce risks, particularly for those new to saunas or hot environments.
Medication Interactions:
It is important to consider that there are life-threatening medication-related hyperthermia syndromes in psychiatry, but that these are not caused by heat exposure (Ahuja & Cole, 2018). These include:
Neuroleptic Malignant Syndrome (NMS)
Serotonin Syndrome
Malignant (or Lethal) Catatonia (often linked to antipsychotics and mood stabilizers)
Anticholinergic Toxicity Syndrome
Stimulant (cocaine, amphetamine) toxicity
Malignant Hyperpyrexia (typically due to anesthetic agents like succinylcholine and inhalational anesthetics)
Parkinsonism–Hyperpyrexia Syndrome (can occur with withdrawal from dopamine agonists or abrupt changes in Parkinson’s medication)
Thyrotoxicosis (Thyroid Storm) (exacerbated by certain medications like amiodarone)
However, other medications can impact heat tolerance and have been associated with higher risk of adverse heat events (heat exhaustion, heat stroke, dehydration) and greater morbidity in the elderly and in people with predisposing cardiovascular conditions according to a limited number of case studies and case-control studies (Bongers et al., 2020; Kalisch Ellett et al., 2016):
Cardiovascular drugs
Diuretics, which increase dehydration risk.
ACE inhibitors, which can impair thirst.
Propranolol, a beta-blocker, increases sweating and therefore fluid replacement is essential.
Vasodilators like nitroglycerin, hydralazine, isosorbide dinitrate lower blood pressure by relaxing blood vessels, which can dissipate body temperature, but can lead to hypotension and syncope.
GLP-1 inhibitors impair thirst.
NSAIDs, potentially through altering prostaglandin signaling, which is known to be important for generating a fever.
Anticholinergics (amitriptyline, oxybutynin, diphenhydramine, benztropine) and drugs with anticholinergic activity (tricyclic antidepressants, clozapine, olanzapine, quetiapine, chlorpromazine, and thioridazine) can impair sweating.
See episode 102 for a deep dive into anticholinergic medications.
Antipsychotics, especially first generation through their high-affinity for D2 receptors, can cause hyperthermia through neuroleptic malignant syndrome (NMS) and potentially through non-NMS mechanisms by affecting the hypothalamus, the body’s principal heat regulator.
Also consider that antipsychotics bind to other sites:
Blockage of alpha-1 adrenergic receptors (especially those with strong sedative properties like chlorpromazine and olanzapine) reduce peripheral vasodilation.
Blocking histamine H1 receptors can also reduce vasodilation.
Clozapine
Fever is a notable side-effect within the first few weeks of initiating the drug, and its prevalence has been suggested to be between 6-60% of patients. It is hypothesized to result from immunomodulatory effects on the hypothalamus (Verdoux et al., 2019). If a patient on clozapine presents with a fever, other adverse clozapine reactions (agranulocytosis, myocarditis) need to be ruled out.
Clozapine is highly anticholinergic, and it is thought that it can reduce sweating in some patients. This anticholinergic effect also leads to constipation and urinary retention. However, clozapine also has a paradoxical agonism at some muscarinic receptors in the body, leading to excessive drooling and, in up to 6% of cases, excessive sweating (Clozaril package insert; Kenton et al., 2023).
SSRIs can impair thermoregulation of the hypothalamus and increase risk of hyponatremia related to SIADH, particularly in the elderly taking fluoxetine or citalopram (Chiu et al., 2020; Kirpekar & Joshi, 2005).
Acknowledgements
We extend our gratitude to Dr. Rhonda Patrick, Dr. Jari Laukkanen, and Dr. Setor Kunutsor for their insightful reviews on sauna and heat therapy (Patrick & Johnson, 2021;Laukkanen & Kunutsor, 2024), which played a crucial role in shaping this episode.
References:
Ahuja, N., & Cole, A. J. (2009). Hyperthermia syndromes in psychiatry. Advances in Psychiatric Treatment, 15(3), 181–191. doi:10.1192/apt.bp.107.005090
Anderson, R. J., Freedland, K. E., Clouse, R. E., & Lustman, P. J. (2001). The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes care, 24(6), 1069–1078. https://doi.org/10.2337/diacare.24.6.1069
Becerra-Tomás, N., Blanco Mejía, S., Viguiliouk, E., Khan, T., Kendall, C. W. C., Kahleova, H., Rahelić, D., Sievenpiper, J. L., & Salas-Salvadó, J. (2020). Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Critical reviews in food science and nutrition, 60(7), 1207–1227. https://doi.org/10.1080/10408398.2019.1565281
Bongers, K. S., Salahudeen, M. S., & Peterson, G. M. (2020). Drug-associated non-pyrogenic hyperthermia: a narrative review. European journal of clinical pharmacology, 76(1), 9–16. https://doi.org/10.1007/s00228-019-02763-5
Chiu, C. Y., Sarwal, A., Azhar Munir, R., Widjaja, M., Khalid, A., & Khanna, R. (2020). Syndrome of Inappropriate Antidiuretic Hormone (SIADH) Induced by Long-Term Use of Citalopram and Short-Term Use of Naproxen. The American journal of case reports, 21, e926561. https://doi.org/10.12659/AJCR.926561
Cornelissen, V. A., & Fagard, R. H. (2005). Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension (Dallas, Tex. : 1979), 46(4), 667–675. https://doi.org/10.1161/01.HYP.0000184225.05629.51
Cozzi, F., Ciprian, L., Carrara, M., Galozzi, P., Zanatta, E., Scanu, A., Sfriso, P., & Punzi, L. (2018). Balneotherapy in chronic inflammatory rheumatic diseases—a narrative review. Int J Biometeorol 62, 2065–2071. https://doi.org/10.1007/s00484-018-1618-z
Enache, D., Winblad, B., & Aarsland, D. (2011). Depression in dementia: epidemiology, mechanisms, and treatment. Current Opinion in Psychiatry 24(6):p 461-472. DOI: 10.1097/YCO.0b013e32834bb9d4
Garcia, L., Pearce, M., Abbas, A., Mok, A., Strain, T., Ali, S., Crippa, A., Dempsey, P. C., Golubic, R., Kelly, P., Laird, Y., McNamara, E., Moore, S., de Sa, T. H., Smith, A. D., Wijndaele, K., Woodcock, J., & Brage, S. (2023). Non-occupational physical activity and risk of cardiovascular disease, cancer and mortality outcomes: a dose-response meta-analysis of large prospective studies. British journal of sports medicine, 57(15), 979–989. https://doi.org/10.1136/bjsports-2022-105669
Genuis, S. J., Birkholz, D., Rodushkin, I., & Beesoon, S. (2011). Blood, urine, and sweat (BUS) study: monitoring and elimination of bioaccumulated toxic elements. Archives of environmental contamination and toxicology, 61(2), 344–357. https://doi.org/10.1007/s00244-010-9611-5
Graham Jr., J. M., Edwards, M. J., & Edwards, M. J. (1998). Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology, 58(5), 209-221. https://doi.org/10.1002/(SICI)1096-9926(199811)58:5<209::AID-TERA8>3.0.CO;2-Q
Hackett, M. L., & Pickles, K. (2014). Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. International journal of stroke : official journal of the International Stroke Society, 9(8), 1017–1025. https://doi.org/10.1111/ijs.12357
Hanusch, K. U., & Janssen, C. W. (2019). The impact of whole-body hyperthermia interventions on mood and depression – are we ready for recommendations for clinical application? International Journal of Hyperthermia, 36(1), 572–580. https://doi.org/10.1080/02656736.2019.1612103
Harris, W. S., Tintle, N. L., Imamura, F., Qian, F., Ardisson Korat, A. V., Marklund, M., Djoussé, L., Bassett, J. K., Carmichael, P., Chen, Y., Hirakawa, H., Küpers, L. K., Laguzzi, F., Lankinen, M., Murphy, R. A., Samieri, C., Senn, M. K., Shi, P., Virtanen, J. K., Brouwer, I. A., ... in collaboration with the Fatty Acids and Outcomes Research Consortium (FORCE). (2021). Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun 12, 2329. https://doi.org/10.1038/s41467-021-22370-2
He, F. J., & MacGregor, G. A. (2002). Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. Journal of human hypertension, 16(11), 761–770. https://doi.org/10.1038/sj.jhh.1001459
Hunt, A. P., Minett, G. M., Gibson, O. R., Kerr, G. K., & Stewart, I. B. (2020). Could Heat Therapy Be an Effective Treatment for Alzheimer's and Parkinson's Diseases? A Narrative Review. Frontiers in physiology, 10, 1556. https://doi.org/10.3389/fphys.2019.01556
Hüppe, M., Müller, J., Schulze, J., Wernze, H., & Ohnsorge, P. (2009). Treatment of patients burdened with lipophilic toxicants: A randomized controlled trial. Activitas Nervosa Superior Rediviva, 51(3-4). http://rediviva.sav.sk/51i34/133.pdf
Janssen, C. W., Lowry, C. A., Mehl, M. R., Allen, J. J. B., Kelly, K. L., Gartner, D. E., Medrano, A., Begay,T. K., Rentscher, K., White, J. J., Fridman, A., Roberts, L. J., Robbins, M. L., Hanusch, K., Cole, S. P., & Raison, C. L. (2016). Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 73(8):789–795. doi:10.1001/jamapsychiatry.2016.1031
Kalisch Ellett, L. M., Pratt, N. L., Le Blanc, V. T., Westaway, K., & Roughead, E. E. (2016). Increased risk of hospital admission for dehydration or heat-related illness after initiation of medicines: a sequence symmetry analysis. Journal of clinical pharmacy and therapeutics, 41(5), 503–507. https://doi.org/10.1111/jcpt.12418
Katschinski, D. M., Wiedemann, G. J., Longo, W., d'Oleire, F. R., Spriggs, D., & Robins, H. I. (1999). Whole body hyperthermia cytokine induction: a review, and unifying hypothesis for myeloprotection in the setting of cytotoxic therapy. Cytokine & growth factor reviews, 10(2), 93–97. https://doi.org/10.1016/s1359-6101(99)00006-4
Kenton, E. M., Zoellner, S. M., & Nelson, L. A. (2023). Diltiazem for clozapine-induced generalized hyperhidrosis. The mental health clinician, 13(4), 193–195. https://doi.org/10.9740/mhc.2023.08.193
Ketelhut, S., & Ketelhut, R. G. (2019). The blood pressure and heart rate during sauna bath correspond to cardiac responses during submaximal dynamic exercise. Complementary Therapies in Medicine, 44, 218-222. https://doi.org/10.1016/j.ctim.2019.05.002
Kirpekar, V. C., & Joshi, P. P. (2005). Syndrome of inappropriate ADH secretion (SIADH) associated with citalopram use. Indian journal of psychiatry, 47(2), 119–120. https://doi.org/10.4103/0019-5545.55960
Kojima, D., Nakamura, T., Banno, M., Umemoto, Y., Kinoshita, T., Ishida, Y., & Tajima, F. (2017). Head-out immersion in hot water increases serum BDNF in healthy males. International Journal of Hyperthermia, 34(6), 834–839. https://doi.org/10.1080/02656736.2017.1394502
Kuan, W. H., Chen, Y. L., & Liu, C. L. (2022). Excretion of Ni, Pb, Cu, As, and Hg in Sweat under Two Sweating Conditions. International journal of environmental research and public health, 19(7), 4323. https://doi.org/10.3390/ijerph19074323
Kukkonen-Harjula, K., Oja, P., Laustiola, K., Vuroi, I., Jolkkonen, J., Siitonen, S., & Vapaatalo, H. (1989). Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Europ. J. Appl. Physiol. 58, 543–550 (1989). https://doi.org/10.1007/BF02330710
Kunutsor, S. K., Khan, H., Laukkanen, T., & Laukkanen, J. A. (2017). Joint associations of sauna bathing and cardiorespiratory fitness on cardiovascular and all-cause mortality risk: a long-term prospective cohort study. Annals of Medicine, 50(2), 139–146. https://doi.org/10.1080/07853890.2017.1387927
Kunutsor, S. K., Khan, H., Zaccardi, F., Laukkanen, T., Willeit, P., & Laukkanen, J. A. (2018). Sauna bathing reduces the risk of stroke in Finnish men and women: A prospective cohort study. Neurology, 90(22), e1937–e1944. https://doi.org/10.1212/WNL.0000000000005606
Kunutsor, S. K., Laukkanen, T., & Laukkanen, J. A. (2017). Frequent sauna bathing may reduce the risk of pneumonia in middle-aged Caucasian men: The KIHD prospective cohort study. Respiratory medicine, 132, 161–163. https://doi.org/10.1016/j.rmed.2017.10.018
Laukkanen, T., Khan, H., Zaccardi, F., & Laukkanen, J. A. (2015). Association Between Sauna Bathing and Fatal Cardiovascular and All-Cause Mortality Events. JAMA Intern Med. 175(4):542–548. doi:10.1001/jamainternmed.2014.8187
Laukkanen, J. A., & Kunutsor, S. K. (2024). The multifaceted benefits of passive heat therapies for extending the healthspan: A comprehensive review with a focus on Finnish sauna. Temperature (Austin, Tex.), 11(1), 27–51. https://doi.org/10.1080/23328940.2023.2300623
Laukkanen, T., Kunutsor, S., Kauhanen, J.,& Laukkanen, J. A. (2017). Sauna bathing is inversely associated with dementia and Alzheimer's disease in middle-aged Finnish men, Age and Ageing, 46(2). 245–249. https://doi.org/10.1093/ageing/afw212
Laukkanen, T., Kunutsor, S. K., Zaccardi, F., Lee, E., Willeit, P., Khan, H., & Laukkanen, J. A. (2018). Acute effects of sauna bathing on cardiovascular function. J Hum Hypertens, 32, 129–138. https://doi.org/10.1038/s41371-017-0008-z
Laukkanen, J. A., Laukkanen, T., & Kunutsor, S. K. (2018). Cardiovascular and Other Health Benefits of Sauna Bathing: A Review of the Evidence. Mayo Clinic proceedings, 93(8), 1111–1121. https://doi.org/10.1016/j.mayocp.2018.04.008
Laukkanen, T., Laukkanen, J. A., & Kunutsor, S. K. (2019). Sauna bathing and risk of psychotic disorders: A prospective cohort study. Medical Principles and Practice, 27(6), 562–569. https://doi.org/10.1159/000493392
Lee, E., Kolunsarka, I., Kostensalo, J., Ahtiainen, J. P., Haapala, E. A., Willeit, P., Kunutsor, S. K., & Laukkanen, J. A. (2022). Effects of regular sauna bathing in conjunction with exercise on cardiovascular function: a multi-arm, randomized controlled trial. American journal of physiology. Regulatory, integrative and comparative physiology, 323(3), R289–R299. https://doi.org/10.1152/ajpregu.00076.2022
Li, Z., Li, Y., Chen, L., Chen, P., & Hu, Y. (2015). Prevalence of Depression in Patients With Hypertension: A Systematic Review and Meta-Analysis. Medicine, 94(31), e1317. https://doi.org/10.1097/MD.0000000000001317
Lichtman, J. H., Bigger Jr., J. T. , Blumenthal, J. A., Frasure-Smith, N., Kaufmann, P. G., Lespéance, F., Mark, D. B., Sheps, D. S., Taylor, C. B., & Froelicher, E. S. (2008). Care and outcomes research: Endorsed by the American Psychiatric Association. Circulation, 118(17). https://doi.org/10.1161/CIRCULATIONAHA.108.190769
Mac Giollabhui, N., Lowry, C. A., Nyer, M., Foster, S. L., Liu, R. T., Smith, D. G., Cole, S. P., Mason, A. E., Mischoulon, D., & Raison, C. L. (2024). The antidepressant effect of whole-body hyperthermia is associated with the classical interleukin-6 signaling pathway. Brain, behavior, and immunity, 119, 801–806. https://doi.org/10.1016/j.bbi.2024.04.040
McGorm, H., Roberts, L. A., Coombes, J. S., & Peake, J. M. (2018). Turning Up the Heat: An Evaluation of the Evidence for Heating to Promote Exercise Recovery, Muscle Rehabilitation and Adaptation. Sports medicine (Auckland, N.Z.), 48(6), 1311–1328. https://doi.org/10.1007/s40279-018-0876-6
Mandsager, K., Harb, S., Cremer, P., Phelan, D., Nissen, S. E., & Jaber, W. (2018). Association of Cardiorespiratory Fitness With Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA Netw Open. 1(6):e183605. doi:10.1001/jamanetworkopen.2018.3605
Masuda, A., Nakazato, M., Kihara, T., Minagoe, S., & Tei, C. (2005). Repeated Thermal Therapy Diminishes Appetite Loss and Subjective Complaints in Mildly Depressed Patients. Psychosomatic Medicine 67(4). 643-647. DOI: 10.1097/01.psy.0000171812.67767.8f
Naumann, J., Grebe, J., Kaifel, S. Weinert,T., Sadaghiani, C., & Huber, R. (2017) Effects of hyperthermic baths on depression, sleep and heart rate variability in patients with depressive disorder: a randomized clinical pilot trial. BMC Complement Altern Med 17, 172. https://doi.org/10.1186/s12906-017-1676-5
Neter, J. E., Stam, B. E., Kok, F. J., Grobbee, D. E., & Geleijnse, J. M. (2003). Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension, 42(5), 878–884. https://doi.org/10.1161/01.HYP.0000094221.86888.AE
Patrick, R. P., & Johnson, T. L. (2021). Sauna use as a lifestyle practice to extend healthspan. Experimental Gerontology, 154, 111509. https://doi.org/10.1016/j.exger.2021.111509
Paz, M. A., de-La-Sierra, A., Sáez, M., Barceló, M. A., Rodríguez, J. J., Castro, S., Lagarón, C., Garrido, J. M. M., Vera, P., & Coll-de-Tuero, G. (2016). Treatment efficacy of anti-hypertensive drugs in monotherapy or combination: ATOM systematic review and meta-analysis of randomized clinical trials according to PRISMA statement. Medicine, 95(30), e4071. https://doi.org/10.1097/MD.0000000000004071
Pizzey, F. K., Smith, E. C., Ruediger, S. L., Keating, S. E., Askew, C. D., Coombes, J. S., & Bailey, T. G. (2021). The effect of heat therapy on blood pressure and peripheral vascular function: A systematic review and meta-analysis. Experimental physiology, 106(6), 1317–1334. https://doi.org/10.1113/EP089424
Puder, D. (Host). (n.d). Anticholinergic Burden (No. 102) [Audio Podcast Episode]. In Psychiatry & Psychotherapy Podcast, Emotion Connection, LLC. https://www.psychiatrypodcast.com/psychiatry-psychotherapy-podcast/anticholinergic
Rissanen, J. A., Häkkinen, A., Laukkanen, J., Kraemer, W. J., & Häkkinen, K. (2020). Acute Neuromuscular and Hormonal Responses to Different Exercise Loadings Followed by a Sauna. Journal of strength and conditioning research, 34(2), 313–322. https://doi.org/10.1519/JSC.0000000000003371
Romeyke, T., & Stummer, H. (2014). Multi-Modal Pain Therapy of Fibromyalgia Syndrome with Integration of Systemic Whole-Body Hyperthermia – Effects on Pain Intensity and Mental State: A Non-Randomised Controlled Study. Journal of Musculoskeletal Pain, 22(4), 341–355. https://doi.org/10.3109/10582452.2014.949336
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P., & Bukau, B. (2019). The Hsp70 chaperone network. Nature reviews. Molecular cell biology, 20(11), 665–680. https://doi.org/10.1038/s41580-019-0133-3
Sacks, F. M., Svetkey, L. P., Vollmer, W. M., Appel, L. J., Bray, G. A., Harsha, D., Obarzanek, E., Conlin, P. R., Miller, E. R., 3rd, Simons-Morton, D. G., Karanja, N., Lin, P. H., & DASH-Sodium Collaborative Research Group (2001). Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine, 344(1), 3–10. https://doi.org/10.1056/NEJM200101043440101
Sebők, J., Édel, Z., Váncsa, S., Farkas, N., Kiss, S., Erőss, B., Török, Z., Balogh, G., Balogi, Z., Nagy, R., Hooper, P. L., Geiger, P. C., Wittmann, I., Vigh, L., Dembrovszky, F., & Hegyi, P. (2021). Heat therapy shows benefit in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group, 38(1), 1650–1659. https://doi.org/10.1080/02656736.2021.2003445
Stetler, R. A., Gan, Y., Zhang, W., Liou, A. K., Gao, Y., Cao, G., & Chen, J. (2010). Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Progress in neurobiology, 92(2), 184–211. https://doi.org/10.1016/j.pneurobio.2010.05.002
Tei, C., Shinsato, T., Miyata, M., Kihara, T., & Hamasaki, S. (2007). Waon therapy improves peripheral arterial disease. Journal of the American College of Cardiology, 50(22), 2169-2171. https://doi.org/10.1016/j.jacc.2007.08.025
Ukai, T., Iso, H., Yamagishi, K., Saito, I., Kokubo, Y., Yatsuya, H., Muraki, I., Eshak, E. S., Sawada, N., & Tsugane, S. (2020). Habitual tub bathing and risks of incident coronary heart disease and stroke. Heart (British Cardiac Society), 106(10), 732–737. https://doi.org/10.1136/heartjnl-2019-315752
U. S. Centers for Disease Control and Prevention (CDC). (2023, June 20). Depression and Aging. https://www.cdc.gov/aging/olderadultsandhealthyaging/depression-and-aging.html
Verdoux, H., Quiles, C., & de Leon, J. (2019). Clinical determinants of fever in clozapine users and implications for treatment management: A narrative review. Schizophrenia Research, 211, 1-9. https://doi.org/10.1016/j.schres.2019.07.040
Windsor, M. T., Bailey, T. G., Perissiou, M., Meital, L., Golledge, J., Russell, F. D., & Askew, C. D. (2018). Cytokine responses to acute exercise in healthy older adults: The effect of cardiorespiratory fitness. Frontiers in Physiology, 9, Article 203. https://doi.org/10.3389/fphys.2018.00203
Xin, X., He, J., Frontini, M. G., Ogden, L. G., Motsamai, O. I., & Whelton, P. K. (2001). Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension (Dallas, Tex. : 1979), 38(5), 1112–1117. https://doi.org/10.1161/hy1101.093424
Ye, W. N., Thipse, M., Mahdi, M. B., Azad, S., Davies, R., Ruel, M., Silver, M. A., Hakami, L., Mesana, T., Leenen, F., & Mussivand, T. (2020). Can heat therapy help patients with heart failure?. Artificial organs, 44(7), 680–692. https://doi.org/10.1111/aor.13659
Yoshihara, T., Naito, H., Kakigi, R., Ichinoseki-Sekine, N., Ogura, Y., Sugiura, T., & Katamoto, S. (2013). Heat stress activates the Akt/mTOR signalling pathway in rat skeletal muscle. Acta physiologica (Oxford, England), 207(2), 416–426. https://doi.org/10.1111/apha.12040
Zaccardi, F., Laukkanen, T., Willeit, P., Kunutsor, S. K., Kauhanen, J., & Laukkanen, J. A. (2017). Sauna Bathing and Incident Hypertension: A Prospective Cohort Study. American Journal of Hypertension, 30(11). 1120–1125. https://doi.org/10.1093/ajh/hpx102